Dr. Daniel Tapken, Dipl.-Biochem., Akad. Rat
Main Research Topic
Plant Glutamate Receptor Homologues
Ionotropic glutamate receptors in animals have been known for decades. In the brains of vertebrates these ligand-gated ion channels play a crucial role in the communication between nerve cells. At the contact site between two cells, the sender cell releases the neurotransmitter glutamate, which in turn activates glutamate receptors at the surface of the receiver cell. Upon activation, the glutamate receptors open their intrinsic ion channel, allowing influx of ions into the cell and thus generating an electrical signal, which can then be further propagated inside the receiver cell.
 In 1998 putative glutamate receptor genes were discovered in the plant Arabidopsis thaliana. This was a surprising finding because plants neither have a brain nor a nervous system. Nevertheless, Arabidopsis has even two glutamate receptor genes more than vertebrates, as it turned out after the sequencing of its genome had been completed in 2000. The proteins coded by these genes are termed glutamate-like receptors (GLRs) and resemble the glutamate receptors from animals in both sequence and predicted structure. This structural similarity suggests that their function may also be similar.
In 1998 putative glutamate receptor genes were discovered in the plant Arabidopsis thaliana. This was a surprising finding because plants neither have a brain nor a nervous system. Nevertheless, Arabidopsis has even two glutamate receptor genes more than vertebrates, as it turned out after the sequencing of its genome had been completed in 2000. The proteins coded by these genes are termed glutamate-like receptors (GLRs) and resemble the glutamate receptors from animals in both sequence and predicted structure. This structural similarity suggests that their function may also be similar.
 Investigating plant GLR function, however, turned out to be remarkably difficult. For long time, only in planta analyses were successful, yet did not allow to directly characterise the molecular properties of single GLR subunits. In some cases, the observed effects could not even be definitely attributed to GLRs at all. Overall, a large variety of possible functions were suggested, including the regulation of carbon and nitrogen metabolism, hormone biosynthesis, water balance, and ion distribution as well as the response to various environmental stress stimuli. However, the analysis of individual, isolated GLR proteins was not successful for long time. Therefore, it was unclear whether they really work in a way similar to glutamate receptors from animals, i. e. opening an intrinsic ion channel upon activation by a transmitter such as glutamate and thus triggering an electrical signal in the cell.
Investigating plant GLR function, however, turned out to be remarkably difficult. For long time, only in planta analyses were successful, yet did not allow to directly characterise the molecular properties of single GLR subunits. In some cases, the observed effects could not even be definitely attributed to GLRs at all. Overall, a large variety of possible functions were suggested, including the regulation of carbon and nitrogen metabolism, hormone biosynthesis, water balance, and ion distribution as well as the response to various environmental stress stimuli. However, the analysis of individual, isolated GLR proteins was not successful for long time. Therefore, it was unclear whether they really work in a way similar to glutamate receptors from animals, i. e. opening an intrinsic ion channel upon activation by a transmitter such as glutamate and thus triggering an electrical signal in the cell.
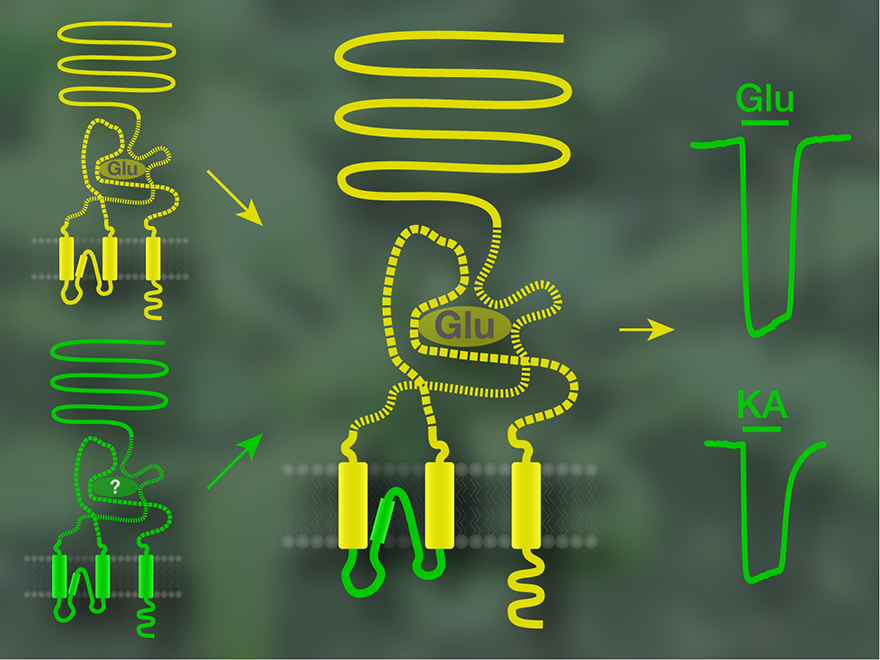 In 2008 we showed that some of the GLR proteins indeed have a functional ion channel. We inserted the part of the plant protein that presumably forms the ion channel into an animal glutamate receptor, replacing its intrinsic channel. The glutamate binding site of the animal glutamate receptor was preserved, so it could still be activated by glutamate. With this approach, we tested the putative ion channel sequences of 17 GLR subunits and in two cases obtained a chimaeric receptor that allowed ion flux upon activation with glutamate. This was clear evidence that GLRs from Arabidopsis can in principle form ion channels.
In 2008 we showed that some of the GLR proteins indeed have a functional ion channel. We inserted the part of the plant protein that presumably forms the ion channel into an animal glutamate receptor, replacing its intrinsic channel. The glutamate binding site of the animal glutamate receptor was preserved, so it could still be activated by glutamate. With this approach, we tested the putative ion channel sequences of 17 GLR subunits and in two cases obtained a chimaeric receptor that allowed ion flux upon activation with glutamate. This was clear evidence that GLRs from Arabidopsis can in principle form ion channels.
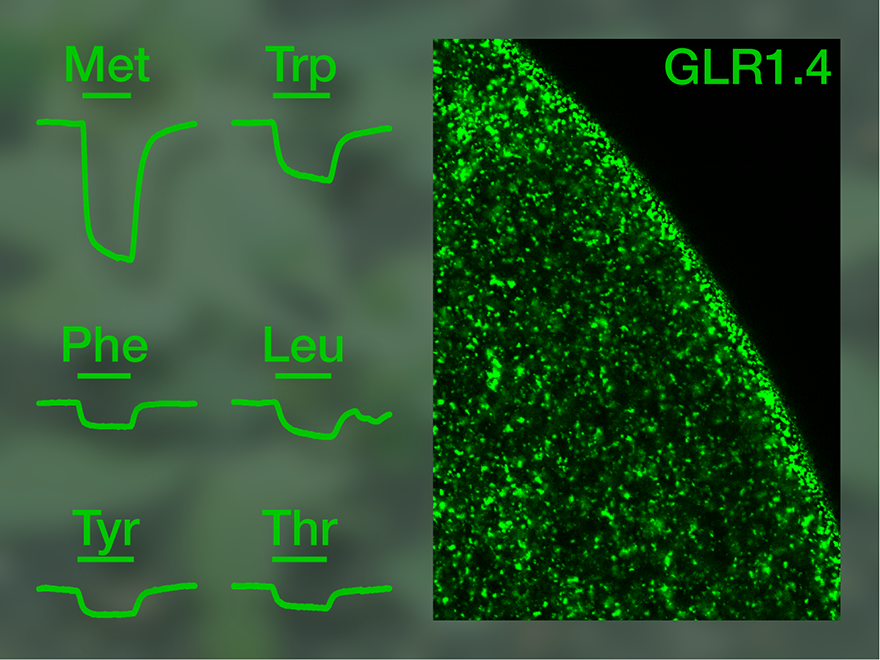 We then concentrated on the two GLR subunits bearing a functional ion channel according to the chimaera study. The full-length plant receptors did not react to glutamate, but one of them was activated by a number of other amino acids, triggering ion flux into the cell. Thus, at least this GLR subunit, GLR1.4, is a ligand-gated ion channel with a mode of operation similar to that of animal glutamate receptors. However, an unusual feature is that not only one defined signalling molecule but several different, yet related substances can activate the receptor, with glutamate being ineffective. This means that GLR1.4 cannot be termed a glutamate receptor. By now, another subunit with similar properties has been identified, which is also an ion channel activated by several amino acids, yet by others than GLR1.4.
We then concentrated on the two GLR subunits bearing a functional ion channel according to the chimaera study. The full-length plant receptors did not react to glutamate, but one of them was activated by a number of other amino acids, triggering ion flux into the cell. Thus, at least this GLR subunit, GLR1.4, is a ligand-gated ion channel with a mode of operation similar to that of animal glutamate receptors. However, an unusual feature is that not only one defined signalling molecule but several different, yet related substances can activate the receptor, with glutamate being ineffective. This means that GLR1.4 cannot be termed a glutamate receptor. By now, another subunit with similar properties has been identified, which is also an ion channel activated by several amino acids, yet by others than GLR1.4.
The majority of plant glutamate receptor homologues, however, still remain completely uncharacterised, their functions in the plant are largely unclear, and the atypical properties of the subunits characterised so far are poorly understood on a molecular level. In our group we are working to answer some of the open questions to contribute to a better understanding of the mysterious family of plant GLR proteins.
Publications
- D.C. Ung, N. Pietrancosta, E.B. Badillo, B. Raux, D. Tapken, A. Zlatanovic, A. Doridant, B. Pode-Shakked, A. Raas-Rothschild, O. Elpeleg, B. Abu-Libdeh, N. Hamed, M.-A. Papon, S. Marouillat, R.-A. Thépault, G. Stevanin, J. Elegheert, M. Letellier, M. Hollmann, B. Lambolez, L. Tricoire, A. Toutain, R. Hepp, and F. Laumonnier (2024).
GRID1/GluD1 homozygous variants linked to intellectual disability and spastic paraplegia impair mGlu1/5 receptor signaling and excitatory synapses.
Molecular Psychiatry 29: 1205-1215.
doi: 10.1038/s41380-024-02469-w
Abstract
- G. Gutti, J. Leifeld, R. Kakarla, N.G. Bajad, A. Ganeshpurkar, A. Kumar, S. Krishnamurthy, C. Klein-Schmidt, D. Tapken, M. Hollmann, and S.K. Singh (2023).
Discovery of triazole-bridged aryl adamantane analogs as an intriguing class of multifunctional agents for treatment of Alzheimer's disease.
European Journal of Medicinal Chemistry 259: 115670.
doi: 10.1016/j.ejmech.2023.115670
Abstract
- M. Masternak, A. Koch, S. Laulumaa, D. Tapken, M. Hollmann, F.S. Jørgensen, and J.S. Kastrup (2023).
Differences between the GluD1 and GluD2 receptors revealed by GluD1 X-ray crystallography, binding studies and molecular dynamics.
FEBS Journal 290(15): 3781-3801.
doi: 10.1111/febs.16631
Abstract
- H. Pan, A.A. Steixner-Kumar, A. Seelbach, N. Deutsch, A. Ronnenberg, D. Tapken, N. von Ahsen, M. Mitjans, H. Worthmann, R. Trippe, C. Klein-Schmidt, N. Schopf, K. Rentzsch, M. Begemann, J. Wienands, W. Stöcker, K. Weissenborn, M. Hollmann, K.-A. Nave, F. Lühder, and H. Ehrenreich (2021).
Multiple inducers and novel roles of autoantibodies against the obligatory NMDAR subunit NR1: a translational study from chronic life stress to brain injury.
Molecular Psychiatry 26: 2471-2482
doi: 10.1038/s41380-020-0672-1
Abstract
- H. Pan, B. Oliveira, G. Saher, E. Dere, D. Tapken, M. Mitjans, J. Seidel, J, Wesolowski, D. Wakhloo, C. Klein-Schmidt, A. Ronnenberg, K. Schwabe, R. Trippe, K. Mätz-Rensing, S. Berghoff, Y. Al-Krinawe, H. Martens, M. Begemann, W. Stöcker, F.-J. Kaup, R. Mischke, S. Boretius, K.-A. Nave, J.K. Krauss, M. Hollmann, F. Lühder, and H. Ehrenreich (2019).
Uncoupling the widespread occurrence of anti-NMDAR1 autoantibodies from neuropsychiatric disease in a novel autoimmune model.
Molecular Psychiatry 24: 1489-1501.
doi: 10.1038/s41380-017-0011-3
Abstract
- J. Borycz, A. Ziegler, J.A. Borycz, G. Uhlenbrock, D. Tapken, L. Caceres, M. Hollmann, B.T. Hovemann, I.A. Meinertzhagen (2018).
Location and functions of Inebriated in the Drosophila eye.
Biology Open 7(7): bio034926.
doi: 10.1242/bio.034926
Abstract
- D. Tapken, T.B. Steffensen, R. Leth, L.B. Kristensen, A. Gerbola, M. Gajhede, F.S. Jørgensen, L. Olsen, and J.S. Kastrup (2017).
The low binding affinity of D-serine at the ionotropic glutamate receptor GluD2 can be attributed to the hinge region.
Scientific Reports 7: 46145.
doi: 10.1038/srep46145
Abstract
- E. Castillo-Gómez, B. Oliveira, D. Tapken, S. Bertrand, C. Klein-Schmidt, H. Pan, P. Zafeiriou, J. Steiner, B. Jurek, R. Trippe, H. Prüss, W.-H. Zimmermann, D. Bertrand, H. Ehrenreich, and M. Hollmann (2017).
All naturally occurring autoantibodies against the NMDA receptor subunit NR1 have pathogenic potential irrespective of epitope and immunoglobulin class.
Molecular Psychiatry 22: 1776-1784.
doi: 10.1038/mp.2016.125
Abstract Press Release
- A.P. Larsen, P. Francotte, K. Frydenvang, D. Tapken, E. Goffin, P. Fraikin, D.H. Caignard, P. Lestage, L. Danober, B. Pirotte, and J.S. Kastrup (2016).
Synthesis and pharmacology of mono-, di- and trialkyl-substituted 7-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxides combined with X-ray structure analysis to understand the unexpected structure-activity relationship at AMPA receptors.
ACS Chemical Neuroscience 7(3): 378-390.
doi: 10.1021/acschemneuro.5b00318
Abstract
- N. Krogsgaard-Larsen, M. Storgaard, C. Møller, C.S. Demmer, J. Hansen, L. Han, R.N. Monrad, B. Nielsen, D. Tapken, D.S. Pickering, J.S. Kastrup, K. Frydenvang, and L. Bunch (2015).
Structure–activity relationship study of ionotropic glutamate receptor antagonist (2S,3R)-3-(3-carboxyphenyl)pyrrolidine-2-carboxylic acid.
Journal of Medicinal Chemistry.
doi: 10.1021/acs.jmedchem.5b00750
Abstract
- S. Pahl, D. Tapken, S.C. Haering, and M. Hollmann (2014).
Trafficking of kainate receptors.
Membranes 4(3): 565-595.
doi: 10.3390/membranes4030565
Abstract
- S.C. Haering, D. Tapken, S. Pahl, and M. Hollmann (2014).
Auxiliary subunits: Shepherding AMPA receptors to the plasma membrane.
Membranes 4(3): 469-490.
doi: 10.3390/membranes4030469
Abstract
- D. Tapken, U. Anschütz, L.-H. Liu, T. Huelsken, G. Seebohm, D. Becker, and M. Hollmann (2013).
A plant homolog of animal glutamate receptors is an ion channel gated by multiple hydrophobic amino acids.
Science Signaling 6(279): ra47.
doi: 10.1126/scisignal.2003762
Abstract Press Release

- A. Orth, D. Tapken, and M. Hollmann (2013).
The delta subfamily of glutamate receptors: characterization of receptor chimeras and mutants.
European Journal of Neuroscience 37(10): 1620-1630.
doi: 10.1111/ejn.12193
Abstract
- T. Huelsken, D. Tapken, T. Dahlmann, H. Wägele, C. Riginos, and M. Hollmann (2012).
Systematics and phylogenetic species delimitation within Polinices s.l. (Caenogastropoda: Naticidae) based on molecular data and shell morphology.
Organisms Diversity and Evolution 12(4): 349-375.
doi: 10.1007/s13127-012-0111-5
Abstract
- E. Wrobel, D. Tapken, and G. Seebohm (2012).
The KCNE tango – how KCNE1 interacts with Kv7.1.
Frontiers in Pharmacology 3: 142.
doi: 10.3389/fphar.2012.00142
Abstract
- G. Seebohm, N. Strutz-Seebohm, O.N. Ursu, R. Preisig-Müller, M. Zuzarte, E.V. Hill, M.-C. Kienitz, S. Bendahhou, M. Fauler, D. Tapken, N. Decher, A. Collins, K. Jurkat-Rott, K. Steinmeyer, F. Lehmann-Horn, J. Daut, J.M. Tavaré, L. Pott, W. Bloch, and F. Lang (2012).
Altered stress stimulation of inward-rectifier potassium channels in Andersen-Tawil syndrome.
The FASEB Journal 26(2): 513-522.
doi: 10.1096/fj.11-189126
Abstract
- N. Strutz-Seebohm, M. Pusch, S. Wolf, R. Stoll, D. Tapken, K. Gerwert, B. Attali, and G. Seebohm (2011).
Structural basis of slow activation gating in the cardiac IKs channel complex.
Cellular Physiology and Biochemistry 27(5): 443-452.
doi: 10.1159/000329965
Abstract
- Z.-L. Ma-Högemeier, C. Körber, M. Werner, D. Racine, E. Muth-Köhne, D. Tapken, and M. Hollmann (2010).
Oligomerization in the endoplasmic reticulum and intracellular trafficking of kainate receptors are subunit- but not editing-dependent.
Journal of Neurochemistry 113(6): 1403-1415.
doi: 10.1111/j.1471-4159.2009.06559.x
Abstract
- C. Sager, D. Tapken, and M. Hollmann (2010).
The C-terminal domains of TARPs: Unexpectedly versatile domains.
Channels 4(3): 155-158.
doi: 10.4161/chan.4.3.11349
Abstract
- N. Strutz-Seebohm, U. Henrion, K. Steinke, D. Tapken, F. Lang, and G. Seebohm (2009).
Serum- and glucocorticoid-inducible kinases (SGK) regulate KCNQ1/KCNE potassium channels.
Channels 3(2): 88-90.
doi: 10.4161/chan.3.2.8086
- S. Kott, C. Sager, D. Tapken, M. Werner, and M. Hollmann (2009).
Comparative analysis of the pharmacology of GluR1 in complex with TARPs γ2, γ3, γ4, and γ8.
Neuroscience 158(1): 78-88 (Special issue “Protein Trafficking, Targeting, and Interaction at the Glutamate Synapse”).
doi: 10.1016/j.neuroscience.2007.12.047
Abstract
- C. Sager, D. Tapken, S. Kott, and M. Hollmann (2009).
Functional modulation of AMPA receptors by transmembrane AMPA receptor regulatory proteins.
Neuroscience 158(1): 45-54 (Special issue “Protein Trafficking, Targeting, and Interaction at the Glutamate Synapse”).
doi: 10.1016/j.neuroscience.2007.12.046
Abstract
- G. Seebohm, N. Strutz-Seebohm, O.N. Ureche, U. Henrion, R. Baltaev, A.F. Mack, G. Korniychuk, K. Steinke, D. Tapken, A. Pfeufer, S. Kääb, C. Bucci, B. Attali, J. Merot, J.M. Tavare, U.C. Hoppe, M.C. Sanguinetti and F. Lang (2008).
Long QT syndrome-associated mutations in KCNQ1 and KCNE1 subunits disrupt normal endosomal recycling of IKs channels.
Circulation Research 103(12): 1451-1457.
doi: 10.1161/circresaha.108.177360
- D. Tapken and M. Hollmann (2008).
Arabidopsis thaliana glutamate receptor ion channel function demonstrated by ion pore transplantation.
Journal of Molecular Biology 383(1): 36-48.
doi: 10.1016/j.jmb.2008.06.076
Abstract
- D. Tapken and M. Hollmann (2006).
Homologs of mammalian glutamate receptors in invertebrates and plants.
In: Biological and biophysical aspects of ligand-gated ion channel receptor superfamilies, H. Arias, ed. Research Signpost, Trivandrum, pp. 321-382.
Abstract
Professional Work
- Since September 2016
- Lecturer at the Department of Biochemistry I – Receptor Biochemistry, Faculty of Chemistry and Biochemistry, Ruhr University Bochum, Germany
- October 2012 to August 2016
- Senior Scientist at the Department of Biochemistry I – Receptor Biochemistry, Faculty of Chemistry and Biochemistry, Ruhr University Bochum, Germany
- October 2011 to September 2012
- Postdoc with a scholarship of the Carlsberg Foundation at the Department of Drug Design and Pharmacology, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark
- October 2010 to September 2011
- Postdoc with a scholarship of the Deutsche Forschungsgemeinschaft at the Department of Medicinal Chemistry, Faculty of Pharmaceutical Sciences, University of Copenhagen, Denmark
- September 2008 to September 2010
- Postdoc at the Department of Biochemistry I – Receptor Biochemistry, Faculty of Chemistry and Biochemistry, Ruhr University Bochum, Germany
Education
- August 2008
- Dr. rer. nat. (Ph.D. in natural sciences) at the Faculty of Chemistry and Biochemistry of the Ruhr University Bochum, Germany
- September 2002 to August 2008
- Doctoral thesis at the Department of Biochemistry I – Receptor Biochemistry, Faculty of Chemistry and Biochemistry, Ruhr University Bochum, Germany
Dissertation: Molecular and functional characterisation of plant glutamate receptors
Advisor: Prof. Dr. Michael Hollmann - July 2002
- Diplom-Biochemiker (M.Sc. in Biochemistry) at the Faculty of Chemistry of the Ruhr University Bochum, Germany
- November 2001 to July 2002
- Diploma (M.Sc.) thesis at the Department of Biochemistry I – Receptor Biochemistry, Faculty of Chemistry, Ruhr University Bochum, Germany
Thesis: Investigation of glutamate receptors from Arabidopsis thaliana
Advisor: Prof. Dr. Michael Hollmann - October 1997 to July 2002
- Studies in Biochemistry at the Ruhr University Bochum, Germany
- May 1996
- Abitur (university-entrance diploma) at the Städtisches Ratsgymnasium, Gladbeck, Germany
Teaching
- Since 2020
- Organisation of the biochemical practicals in the bachelor course of studies in biochemistry
- Since 2017
- Lecture “Molecular Genetic Methods in Biochemistry” in the bachelor course of studies in biochemistry (4th semester)
- Since 2016
- Elective lab course “Methods of Cell Culture and Confocal Microscopy” in the bachelor course of studies in biochemistry (5th semester)
- Since 2014
- Lab course in the bachelor course of studies in biochemistry (5th semester): Lecture, seminar, and demonstration of methods
- Since 2013
- Lecture “Biochemistry II – Biochemistry of Nucleic Acids” in the bachelor course of studies in biochemistry (4th semester)
- Biochemical seminar in the biochemistry master's programme (1st semester)
- 2012
- Ph. D. course „Receptor Structure and Function“: Workshop on differential scanning fluorimetry
- Since 2004
- Supervision of bachelor's and master's theses
- 2003 to 2010
- Lab course in the bachelor course of studies in biochemistry (5th semester): Lecture and demonstration of methods
- Since 2001
- Modular advanced practical “Heterologous Expression of Neurotransmitter Receptors in Frog Oocytes” in the biochemistry master's programme (1st semester), since 2008 including lectures on the theoretical background and the applied techniques
Other work
- 2013 to 2023
- Photographer at the graduation ceremony of the Faculty of Chemistry and Biochemistry
- Since 2003
- Maintenance of the website of the Department of Biochemistry I, 2010 complete redesign and switch to the new corporate design of the Ruhr University, 2016 redesign and since then maintenance of the website of the Department of Biochemistry II
- August 1996 to August 1997
- Civilian service in the mobile nursing service of the Arbeiterwohlfahrt (Workers’ Welfare Association), Gladbeck, Germany
Personal Data
- Date of Birth
- 22 February 1977
- Place of Birth
- Bottrop, Germany