Publication of the Year 2013
D. Tapken, U. Anschütz, L.-H. Liu, T. Huelsken, G. Seebohm, D. Becker, and M. Hollmann (2013).
A plant homolog of animal glutamate receptors is an ion channel gated by multiple hydrophobic amino acids.
Science Signaling 6(279): ra47.
doi: 10.1126/scisignal.2003762
Abstract
Press Release

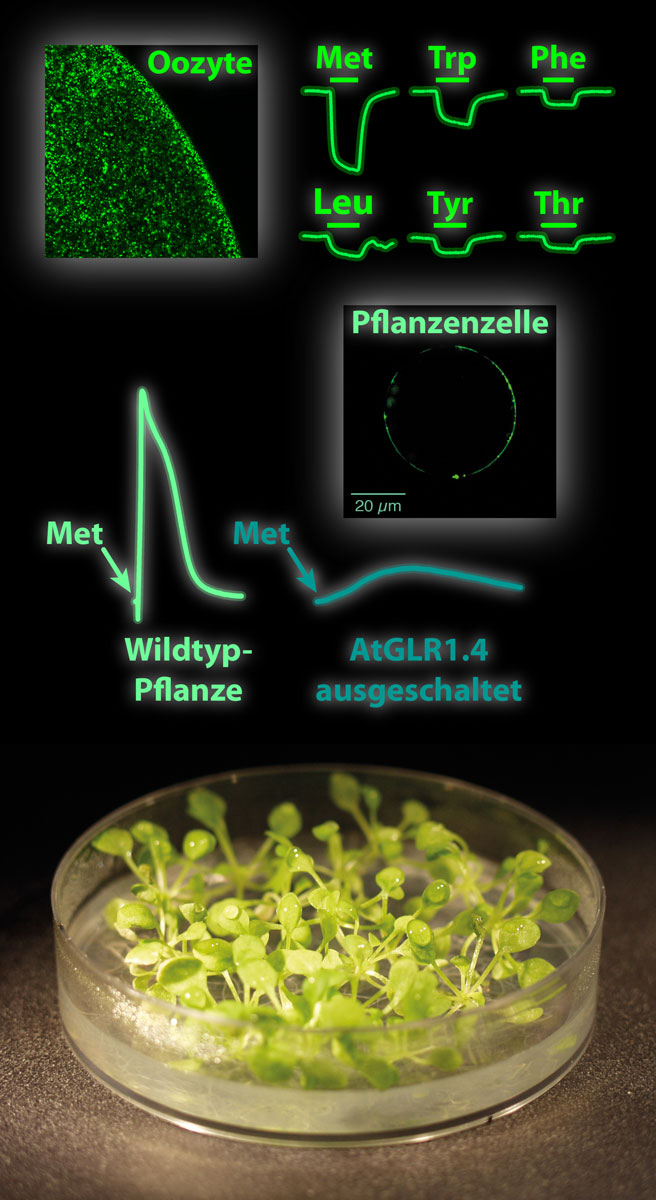 The sequencing of the first plant genome in 2000 led to the unexpected discovery of 20 genes in Arabidopsis thaliana that are homologous to the 18 ionotropic glutamate receptor genes found in mammals. Those genes were named glutamate-like receptors (GLRs). In this study we showed for the first time true ion channel function in a heterologous system for one of the proteins encoded by these genes, AtGLR1.4, and we were able to clarify the nature of the ligand. The surprising result was that none of the known glutamatergic agonists activates the receptor; however, the amino acid methionine as well as six additional, mainly hydrophobic amino acids induce ionic currents and thus act as agonists. Nine other amino acids act as antagonists, while the remaining four amino acids gluta- mate, aspartate, glycine, and proline neither activate nor inhibit the receptor. The physiological relevance of the methionine response was demonstrated via two AtGLR1.4 knockout mutants that, as expected, lacked the methionine response of wild type seedlings.
The sequencing of the first plant genome in 2000 led to the unexpected discovery of 20 genes in Arabidopsis thaliana that are homologous to the 18 ionotropic glutamate receptor genes found in mammals. Those genes were named glutamate-like receptors (GLRs). In this study we showed for the first time true ion channel function in a heterologous system for one of the proteins encoded by these genes, AtGLR1.4, and we were able to clarify the nature of the ligand. The surprising result was that none of the known glutamatergic agonists activates the receptor; however, the amino acid methionine as well as six additional, mainly hydrophobic amino acids induce ionic currents and thus act as agonists. Nine other amino acids act as antagonists, while the remaining four amino acids gluta- mate, aspartate, glycine, and proline neither activate nor inhibit the receptor. The physiological relevance of the methionine response was demonstrated via two AtGLR1.4 knockout mutants that, as expected, lacked the methionine response of wild type seedlings.