Disentangling One- and Two-dimensional Confinement Effects from Wall Effects
(Vladimir Lykov)
This project aims to investigate the effect of confinement on chemical reactions. Confinement increases the collision probability, but it might limit the flexibility of reactants during a chemical reaction. A surface science approach can study this effect of the confinement to one or two dimensions. In this project, we use low temperature (T = 7 K) scanning tunneling microscopy in ultra-high vacuum conditions (base pressure > 10-10 mbar) to study the effect of confinement dimensionality by consecutive adsorption of salt layers, carbon dioxide CO2, and electron-rich phosphines [1,2] onto a metal surface (Fig. 1). The CO2 capturing with electron-rich phosphines will be used in this research as an example of a chemical reaction.
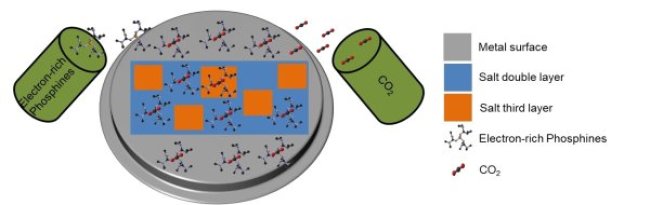
Fig. 1. Schematic drawing of the investigating system
This work was supported by the Research Training group ´Confinement-controlled
Chemistry´, funded by the Deutsche Forschungsgemeinschaft (DFG) under Grant GRK2376
/ 331085229
References
[1] Wünsche, M.A., Mehlmann, P., Witteler, T., Buß, F., Rathmann, P. and
Dielmann, F. Angew. Chem. Int. Ed., 54: 11857-11860 (2015).
[2] Buß, F., Mehlmann, P., Mück-Lichtenfeld, C., Bergander, K., Dielmann, F. J.
Am. Chem. Soc. 138 (6), 1840-1843 (2016)
[3] Vyshnepolsky, M., Morgenstern, K. Phys Chem Chem Phys. 22(2), 497-506
(2020)

