Valorization of Renewables via Isomerizing Metathesis
Synthetic impact of isomerizing metathesis
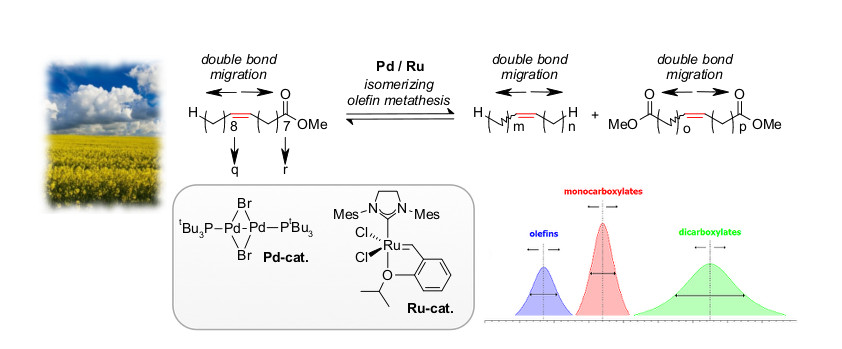
Within the last years, the Gooßen group has established powerful bimetallic catalyst systems consisting of the Pd(I)-based isomerization catalyst [Pd(µ-Br)(tBu3P)]2 and state-of-the-art metathesis catalyst. In the presence of this catalyst system, the double bonds of unsaturated substrates are continuously migrated along the alkyl chains, while at the same time, metathesis reactions occur regardless of the actual position of the double bond. It allows converting mixtures of two different alkenes into homogeneous distributions with adjustable medium chain lengths. This way, fatty acid derivatives have been converted into valuable olefins, mono- or dicarboxylic acids. The concept has also been employed to convert naturally occurring allyl arenes into vinyl arenes, and was utilized in a concise synthesis of tsetse fly attractants from cardanol, a component of cashew nutshell oil.
Isomerizing Olefin Metathesis as a Strategy to Access Defined Distributions of Unsaturated Compounds from Fatty Acids
In the presence of bifunctional catalyst systems consisting of [Pd(μ-Br)tBu3P]2 and NHC–indylidene ruthenium complexes, the isomerizing olefin metathesis becomes an efficient way to access industrially useful multi-component blends from renewable feedstock. Technical quality fatty acids can be employed in isomerizing self-metathesis, ethenolysis, or cross-metathesis reactions, leading to olefin blends of tailored medium chain lengths from renewable resources rather than from crude oil. The mono- and dicarboxylates formed as secondary products create additional value when used as polymer building blocks.
The cross-metathesis of two olefins with different chain lengths leads to regular distributions with a mean chain length that depends on the chain length of both starting materials and their ratio. The cross-metathesis of oleic acid with ethylene serves to access olefin blends with mean chain lengths below 18 carbons, while its analogous reaction with hex-3-enedioic acid gives unsaturated dicarboxylic acids with adjustable mean chain lengths as major products.
Key reference:
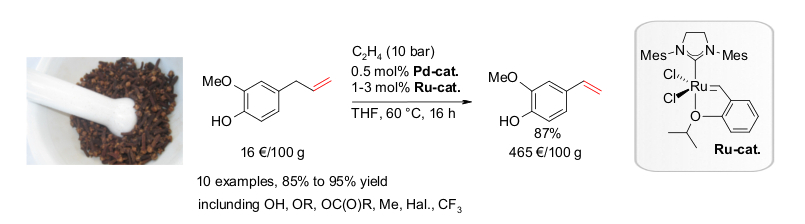
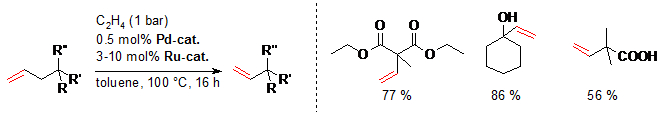
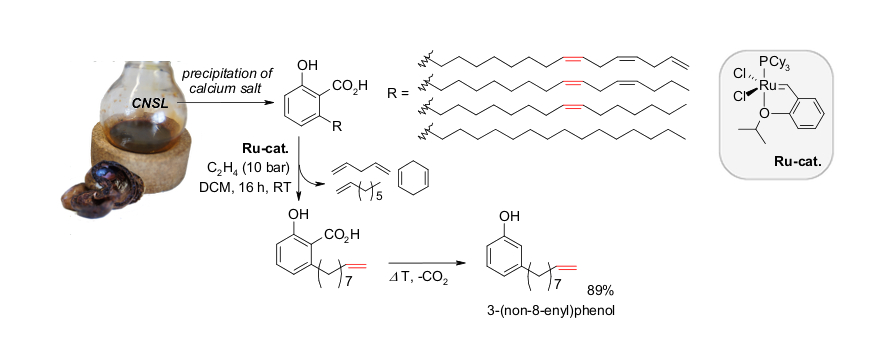
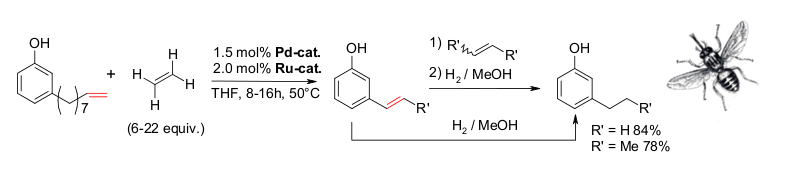
D. M. Ohlmann, N. Tschauder, J.-P. Stockis, K. Gooßen, M. Dierker, L. J. Gooßen, J. Am. Chem. Soc. 2012, 134, 13716-13729. The combination of the Pd(I)-isomerization catalyst with a Hoveyda-Grubbs II catalyst has been found to efficiently promote the cross-metathesis between substituted alkenes and ethylene, while continuously migrating the double bond along the alkenyl chain. When natural products such as eugenol, safrol or estragol were treated with this catalyst system under ethylene pressure, they were cleanly converted into the corresponding styrenes along with propylene gas. The present protocol allowed converting easily accessible molecules with allyl-substituted quaternary carbons into the corresponding vinyl derivatives. Examples include allylated malonates, homoallyl alcohols and unsaturated carboxylic acids. This indicates that isomerizing metatheses may evolve into a generally applicable synthetic tool to introduce vinyl groups into organic molecules. This strategy benefits from the broad spectrum of efficient chemo-, regio- and stereoselective allylation methods. Baader, D. M. Ohlmann, L. J. Gooßen, Chem. Eur. J. 2013, 19, 9807–9810. Isomerizing olefin cross-metathesis enabled the synthesis of the tsetse fly attractants 3-ethylphenol and 3-propylphenol from an anacardic acid mixture extracted from cashew nut shells, obtained as by-product of cashew nut processing. The key towards providing a uniform substrate was the ethenolysis of the anacardic acids followed by thermal decarboxylation. This way, mono-, di- and tri-unsaturated side chains are all shortened to nine carbons, and the 3-(non-8-enyl)phenol was separated from derivatives with saturated side chains. A one-pot sequence consisting of isomerizing ethenolysis followed by an optional butenolysis and hydrogenation of the olefinic side chain furnished the desired 3-propylphenol either in pure form or as a mixture with 3-ethylphenol. S. Baader, P. E. Podsiadly, D. J. Cole-Hamilton, L. J. Gooßen, Green Chem. 2014, 16, 4885-4890.
Isomerizing Ethenolysis as an Efficient Strategy for Styrene Synthesis
Key reference:
Synthesis of Tsetse Fly Attractants from a Cashew Nut Shell Extract by Isomerizing Metathesis
Key reference: