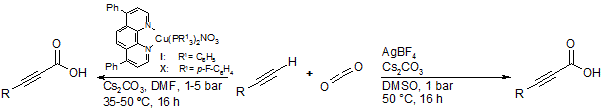Catalytic carboxylation reactions
C-H Carboxylation of terminal alkynes with carbon dioxide
Carbon dioxide is arguably the most attractive C1 building block for organic synthesis as it is the most abundant carbon feedstock in the atmosphere. Therefore we are looking for catalytic transformations that allow us to utilize carbon dioxide.
For that reason we developed coinage metal catalyzed C-H carboxylation´s of terminal alkynes that give access to propiolic acids, which are valuable synthetic intermediates for organic syntheses. In the former protocol copper(I) phenanthroline complexes were used to coordinate to the alkyne and thus activate the terminal C-H bond. Further developments lead to a highly active catalyst system that allowed us to utilize only ppm-quantities of simple silver(I) salts in combination with DMSO.
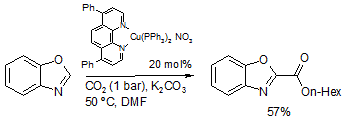
Catalytic carboxylation of heteroarenes
The copper-catalyzed protocol could also be expanded to the carboxylation of heteroarenes with C-H bonds that have a similar pKa to alkynes.
Key References:
| 1. | |
| 2. | |
| 3. |