New Catalytic Transformations - On the Way to "Dream Reactions"
Philosophy and main concepts
Our research is devoted to the development of straightforward catalytic “dream-reactions” as alternatives to inconvenient and hazardous multistep transformations. We are particularly interested in defining new strategies for the minimization of waste produced within a synthetic sequence by:
- utilizing unusual substrate classes for catalytic cross-couplings
- activating inert molecules such as carbon dioxide
- regioselectively functionalizing C-C, C-O or C-H bonds
- designing regio- and stereoselective catalytic addition reactions
Or reaction portfolio includes homogeneous and heterogeneous catalysis, electrochemistry and photochemistry. In the context of catalytic method development, we design novel catalysts and construct customized reactors as well as hard- and software tools for instrumental analysis. Our experimental work is supported by mechanistic investigations and quantum mechanical calculations, and complemented by organic syntheses that allow verifying the practical applicability of the new processes in the preparation of complex molecules such as pharmaceuticals.
Research Cooperations
- Multiple cooperations within the Transregional Collaborative Research Centre 88 „TRIMET“: Cooperative Effects in Homo and Heterometallic Complexes
- Research cooperations with BASF, Bayer, Pfizer, Saltigo, AstraZeneca, Cognis, Novartis, Umicore
- Prof. Dr. G. Röschenthaler, Jakobs Universität Bremen, Development of trifluoromethylation reagents
- Prof. Dr. Nuria Rodríguez Garrido, Universidad Autónoma de Madrid, Spain: Activation of C-O bonds
- Prof. Dr. O. De Lucchi, University of Venice, Italy: Decarboxylative cross-couplings for cyclotrimerizations
- Prof. Dr. A. Vidal, ICIQ Taragona, Spain: Spectroscopic studies of catalytic cross-couplings/li>
- Prof. Dr. A. R. Ferwanah, Universität Gaza, Palestine: Structure elucidation of nitrogen heterocycles
- Prof. Dr. P. Härter, TU München: Carbocyclic Pd-carbenekomplexes in catalysis
- Prof. Dr. W. F. Maier, Universität Saarbrücken: Decarboxylative ketonizations
- Prof. Dr. M. T. Reetz, MPI Mülheim: Asymmetric hydrogenations of enol ethers
- Prof. Dr. W. Thiel, MPI Mülheim: Mechanistic studies of cross-coupling reactions
- Prof. Dr. W. R. Thiel, TU Kaiserslautern: Synthesis and applications of magnetit nanoparticles
- Prof. Dr. G. Niedner-Schatteburg, TU Kaiserslautern: Spectroscopic investigations of Ru-catalyzed addition reactions
- Prof. Dr. J. Hartung, TU Kaiserslautern: Catalytic transformations in microwave reactors
Carboxylic acids as substrates for catalytic transformations
The below scheme nicely demonstrates the main features of cross-coupling reactions and explains why these transformations are so widely used in organic synthesis: Two complex molecules are regioselectively connected at a position defined by the two leaving groups A and B. Ideally, the reaction also occurs highly chemoselective, so that the other functionalities C-G remain unchanged. It is also visible from the scheme, that a cross coupling reaction inevitably leads to the formation of a byproduct AB, in the case of popular C-C bond forming reactions usually a salt. For this reason, the development of waste-free cross-couplings appeared to be impossible. However, with the help of highly reactive catalysts that can activate even s bonds of groups that usually not considered to be leaving groups, the nature of this byproduct can be broadly varied opening up new perspectives for the development of environmentally benign processes.

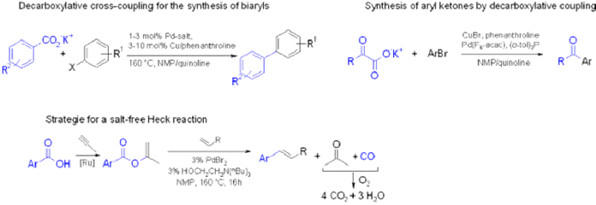
In this context, we have mainly focused on the development of cross-coupling reactions starting from carboxylic acids. Following their in situ activation with peptide coupling reagents, carboxylic acids can oxidatively add to transition metal catalysts and thus replace ecologically questionable organohalides in cross-coupling reactions. On the other hand, their metal salts can be converted into even more valuable carbon nucleophiles via metal-mediated decarboxylation. The new methods resulting from this research allow the straightforward and waste-minimized production of biologically active molecules with structural elements including aryl ketones, arylacetic acids, vinyl arenes, enol esters, and (thio)enamides. We have also developed decarbonylative Heck olefinations, in which phenyl or isopropenyl esters are employed instead of the commonly used acyl or aryl halides, so that solely recyclable organic molecules such as phenols or acetone are formed as byproducts, and no alkali salts are released.
A highlight in this context was the development of decarboxylative cross-coupling reactions: Our biaryl synthesis makes use of inexpensive salts of aromatic carboxylic acids instead of organometallic reagents (e.g. boronic acids). Following the release of carbon dioxide gas, they cross-couple with aryl halides under formation of the biaryl products. An analogous protocol allows the synthesis of aryl ketones from a-oxocarboxylate salts and aryl halides.