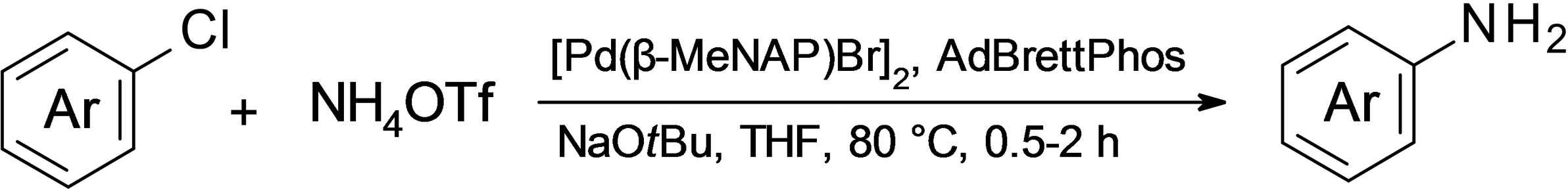
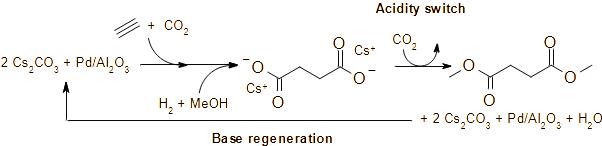
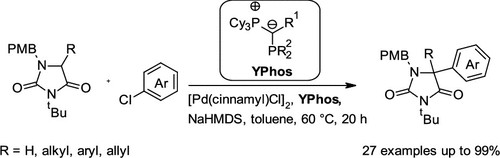
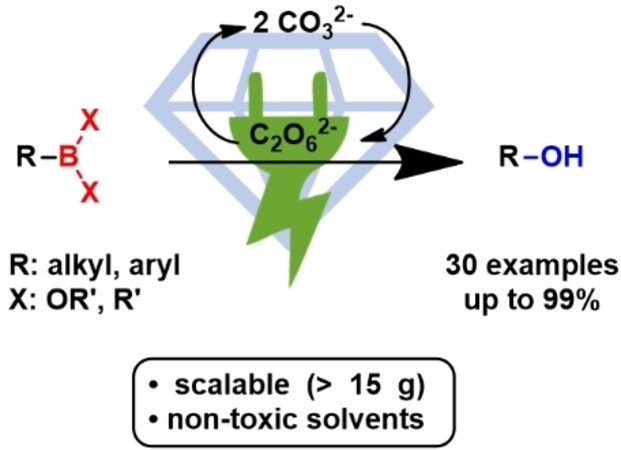
Publications and Patents
| ResearcherID: L-9764-2015 | Google Scholar profile |
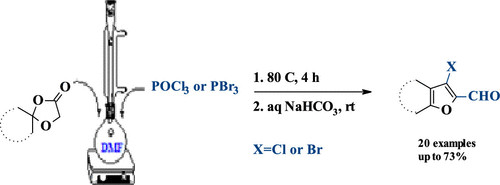
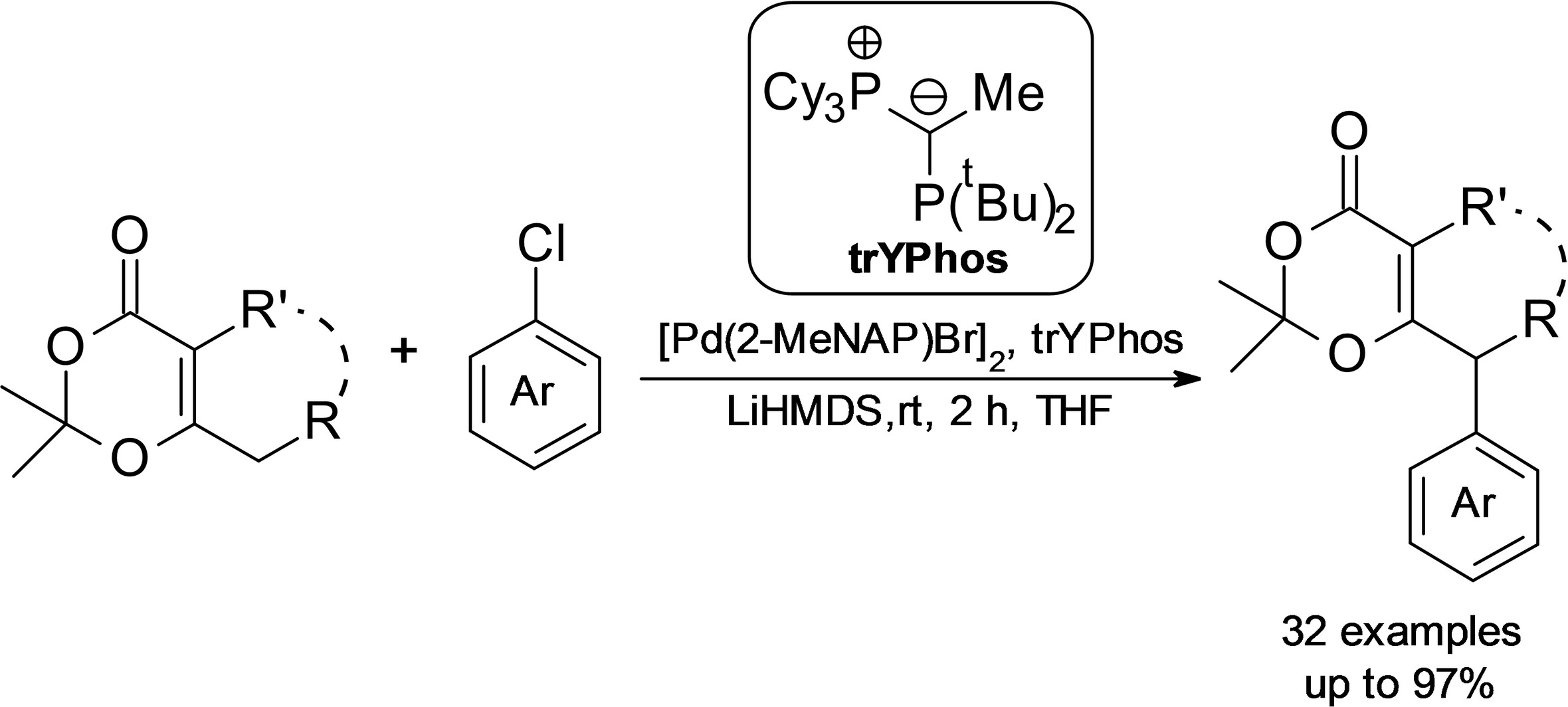
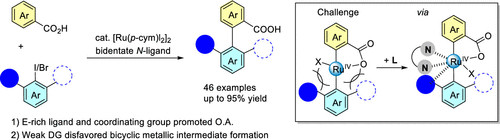
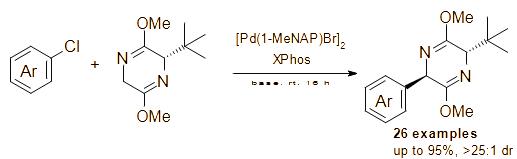
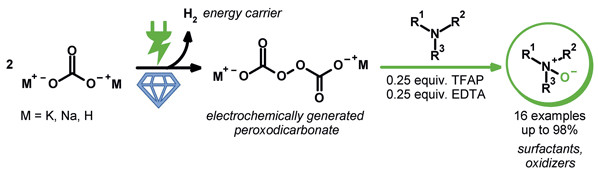
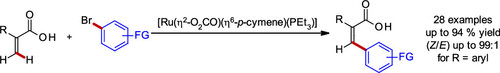
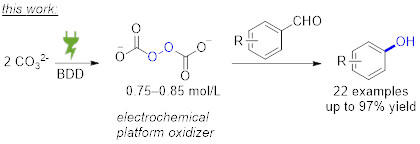
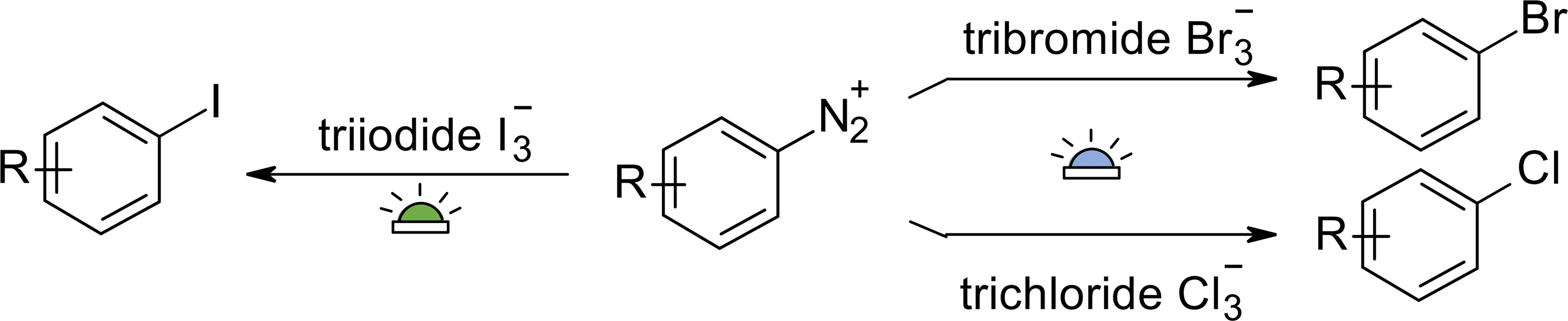
2025
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2024
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023
2022
2021
2020
2019
2018
2017
|
L. J. Gooßen, K. F. Pfister, S. Baader, Patent WO 2017158060A1, 2017: Biofuel and method for a preparation by isomerizing metathesis
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2016
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2015
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2014
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2013
|
|
|
|
|
|
|
|
|
|
|
|
2012
2011
2010
2009
|
L. J. Gooßen, K. Gooßen, Aktuelle Wochenschau der GDCh, 2008, 18: Dream Reactions – Nachhaltigkeit durch atomökonomische Synthesen
|
|
2007
2006 and earlier
|
L. J. Gooßen, J. Paetzold, WO 03/043958, 2003: Verfahren zur Herstellung von Vinylarenen und 1,3-Dienen aus Aryl- bzw. Vinylcarbonsäurederivaten
|
|
|
L. J. Gooßen, K. Baumann, Chemie in unserer Zeit, 2001, 35, 402-403: Asymmetrische Katalyse
|
|
|
L. J. Gooßen, K. Ghosh (Studiengesellschaft Kohle m.b.H.), WO 02/092547, 2002: Verfahren zur Herstellung von Ketonen aus Carbonsäureanhydriden
|
|
|
L. J. Gooßen (Studiengesellschaft Kohle m.b.H.), WO 02/072524, 2002: Verfahren zur Herstellung von Vinyl-, Aryl- und Heteroarylessigsäuren und ihrer Derivate
|
|