Matrix Isolation
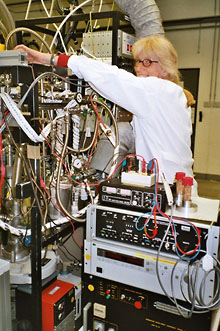
The matrix isolation technique was first introduced in 1954 by Pimentel and co-workers,[1] who used the technique for systematic studies of free radicals and other unstable or transient species. Matrix isolation was developed independently by Norman and Porter[2], and it is used for trapping and producing chemical species and preserving them in solidified inert (or occasionally reactive) gases at low temperatures between 10 – 40 K. The matrices are formed, most of the time, by a non-reactive substance such as rare gases or solid nitrogen. The low temperatures required are achieved by cryostats with closed helium-cycles.
 Since solidified inert gases are used as matrix, interactions between the reaction medium and the molecules to be studied are weak. To avoid reactions between isolated molecules, the samples are highly diluted (1000 : 1) during the preparation of the matrix, resulting in molecules that are spatially separated while embedded into the matrix lattice. In most cases, rearrangements of trapped molecules are avoided at 10 K by sufficiently high energy barriers.
Since solidified inert gases are used as matrix, interactions between the reaction medium and the molecules to be studied are weak. To avoid reactions between isolated molecules, the samples are highly diluted (1000 : 1) during the preparation of the matrix, resulting in molecules that are spatially separated while embedded into the matrix lattice. In most cases, rearrangements of trapped molecules are avoided at 10 K by sufficiently high energy barriers.
For preparing the matrices, the substance or a suitable precursor is condensed simultaneously with an excess of inert gas onto a cooled spectroscopic window (i.e. CsI for IR spectroscopy and a quartz or sapphire window for UV/Vis spectroscopy). Solids and liquids should have a sufficient vapor pressure (about 10-6 mbar) at temperatures which will not lead to decomposition. Gases can be mixed with argon in an appropriate ratio before the deposition is performed.
By codeposition of more than one substance, controlled reactions under matrix conditions can be achieved. The matrix should have a temperature that is about 30 % of the melting point of the noble gas (i. e. 30 K for Argon). Under these conditions, smaller molecules like ozone, carbon monoxide or oxygen are able to diffuse through lattice gaps to encounter a reaction partner. Another kind of reactions of matrix-isolated molecules are photochemically induced processes by irradiation at a suitable wavelength using mercury high pressure lamps or lasers.
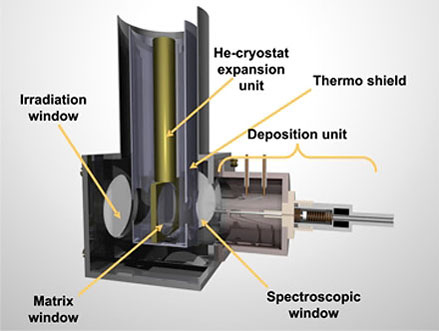
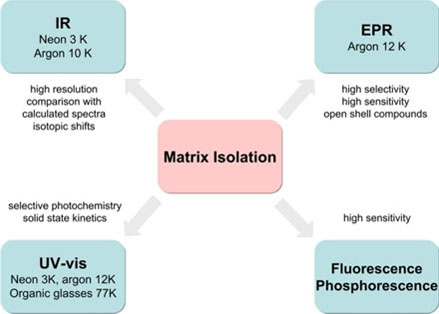 In order to characterize matrix isolated species, infrared, UV/VIS, and EPR spectroscopy are frequently used. Matrix isolation experiments allow one, among other applications, to study unstable molecules generated by photolysis or gas phase thermolysis, to observe directly reaction intermediates, to generate and to study novel reactive species, to determine the structures of reactive species and to freeze out and to study particular molecular conformations. An example for the latter is cyclohexane in its chair and twist conformation.[3]
In order to characterize matrix isolated species, infrared, UV/VIS, and EPR spectroscopy are frequently used. Matrix isolation experiments allow one, among other applications, to study unstable molecules generated by photolysis or gas phase thermolysis, to observe directly reaction intermediates, to generate and to study novel reactive species, to determine the structures of reactive species and to freeze out and to study particular molecular conformations. An example for the latter is cyclohexane in its chair and twist conformation.[3]
 Another important application of the matrix isolation technique is the study of weakly bound systems like H-bonded, charge transfer, and van der Waals complexes that can be isolated in low temperature matrices, despite the fact they dissociate under normal temperature conditions due to the weak intermolecular forces. The IR bands of the components of these complexes are significantly perturbed which provides insight into the intermolecular interactions.[1-4]
Another important application of the matrix isolation technique is the study of weakly bound systems like H-bonded, charge transfer, and van der Waals complexes that can be isolated in low temperature matrices, despite the fact they dissociate under normal temperature conditions due to the weak intermolecular forces. The IR bands of the components of these complexes are significantly perturbed which provides insight into the intermolecular interactions.[1-4]
[1] E. Whittle, D. A. Dows, G. C. Pimentel, J. Chem. Phys. 1954, 22, 1943.
[2] I. Norman, G. Porter, Nature 1954, 174, 508.
[3] M. Squillacote, R. S. Sheridan, O. L. Chapman, F. A. L. Anet, J. Am. Chem. Soc. 1975, 97, 3244.
[4] M. J. Almond, K. S. Wiltshire, Annual Reports on the Progress of Chemistry, Section C: Physical Chemistry 2001, 97, 3.