Welcome to Physical organic chemistry
News Highlights
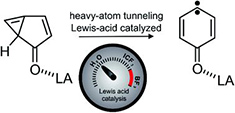
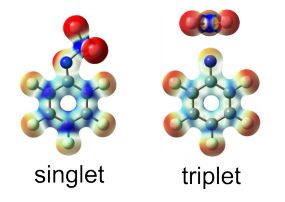
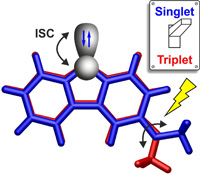
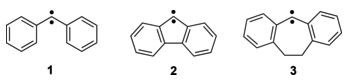
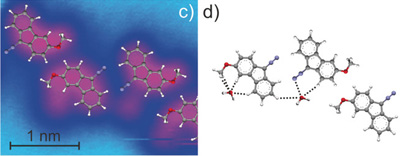
Heavy-atom quantum tunnelling catalysed with Lewis acids
Read the News Article in Chemistry World
Read our publication in Chemical Science
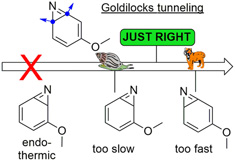
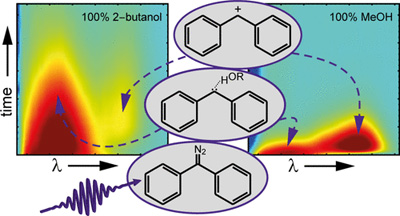
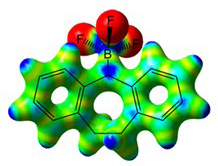
Our recent paper was mentioned in the magazine "Chemistry Views"
Read the article in Chemistry Views