microsolvation
 The majority of chemical reactions, including those that are absolutely central to many important industrial processes and virtually all biological processes, take place in a liquid-state environment. Solvents - with water being the most prominent - are used to “solvate” molecular species from reagents to proteins and thereby transfer these as “solutes” into the liquid state. Solvents also wet extended surfaces such as lipid membranes or metal electrodes, thus creating interfaces. Understanding solvation within the framework of chemistry, physics, and engineering is expected to stimulate major advances in emerging key technologies such as green chemistry and electrochemistry. Additionally, it is a precondition for optimizing industrial processes, avoiding environmental hazards, preventing corrosion, or increasing energy efficiency to name but a few key challenges relevant to society. In the life sciences, understanding the function of water as the ubiquitous solvent, sometimes even called the “matrix of life”, is now recognized to be crucial for comprehensively unravelling biological functions.
The majority of chemical reactions, including those that are absolutely central to many important industrial processes and virtually all biological processes, take place in a liquid-state environment. Solvents - with water being the most prominent - are used to “solvate” molecular species from reagents to proteins and thereby transfer these as “solutes” into the liquid state. Solvents also wet extended surfaces such as lipid membranes or metal electrodes, thus creating interfaces. Understanding solvation within the framework of chemistry, physics, and engineering is expected to stimulate major advances in emerging key technologies such as green chemistry and electrochemistry. Additionally, it is a precondition for optimizing industrial processes, avoiding environmental hazards, preventing corrosion, or increasing energy efficiency to name but a few key challenges relevant to society. In the life sciences, understanding the function of water as the ubiquitous solvent, sometimes even called the “matrix of life”, is now recognized to be crucial for comprehensively unravelling biological functions.
More information: Solvation Science @ RUB
Noncovalent Reactions
The folding of proteins and the formation of molecule crystals is governed by weak intermolecar interactions. Both processes are still not predictable and thus a challenge for experimental and theoretical chemistry. Small molecules can serve as models to understand these complex phenomena.
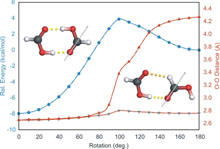
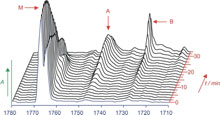
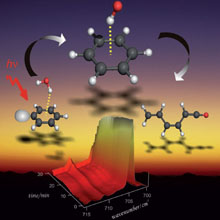
The dimerization of formic acid as an example of a „non-covalent“ reaction that can be directly monitored in solid argon by IR spectroscopy.
Solvation of Diphenylcarbene
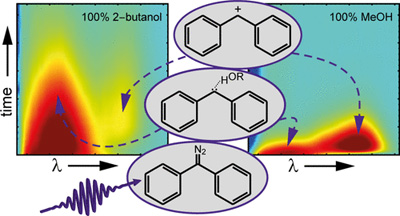
How Protic Solvents Determine the Reaction Mechanisms of Diphenylcarbene in Solution
J. Knorr, P. Sokkar, P. Costa, W. Sander, E. Sanchez-Garcia, P. Nuernberger, J. Org. Chem. 84 (2019), 11450-11457.
We investigate the effects of small admixts. of protic solvent molecules, such as water and alcohols, on the ultrafast dynamics of diphenylcarbene in acetonitrile at room temperature. Broadband transient absorption measurements and quantum mechanics/molecular mechanics molecular dynamics simulations allow elucidating the dominant reaction mechanism of an intermediate hydrogen-bonded complex between singlet diphenylcarbene and a protic solvent mol., thus competing with intersystem crossing. Anal. of the data indicates that complex formation is a diffusion-controlled process with orientational requirements.
Conformation and Dynamics of a Cyclic Disulfide-Bridged Peptide: Effects of Temperature and Solvent
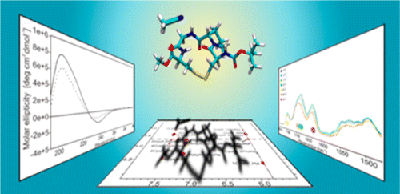
Conformation and Dynamics of a Cyclic Disulfide-Bridged Peptide: Effects of Temperature and Solvent
F. Li, K. Bravo-Rodriguez, C. Phillips, R. W. Seidel, F. Wieberneit, R. Stoll, N. L. Doltsinis, E. Sanchez-Garcia, W. Sander, J. Phys. Chem. B 117 (2013), 3560-3570.
The cyclic disulfide-bridged tetrapeptide cyclo(Boc-Cys-Pro-Gly-Cys-OMe) (1) was designed as a model for the study of solvent-driven conformational changes in peptides. The three-dimensional structure and dynamics of 1 were studied using a variety of experimental and computational techniques.
Read our publication in J. Phys. Chem. B
Phenoxyl Radical-Water Complex
The Phenoxyl Radical–Water Complex—A Matrix Isolation and Computational Study
W. Sander, S. Roy, I. Polyak, J. M. Ramirez-Anguita, E. Sanchez-Garcia, J. Am. Chem. Soc. 134 (2012), 8222-8230.
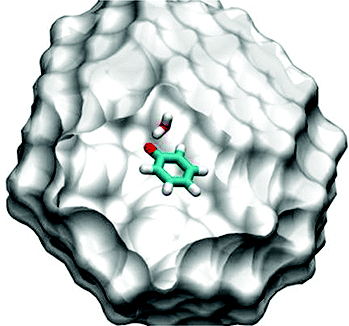 The phenoxyl radical 1 was generated in high yields by flash vacuum pyrolysis of allyl phenyl ether 2 with subsequent trapping of the products in argon at 3 K. In water-doped argon matrices, an OH···O complex between 1 and water is formed that could be characterized by IR spectroscopy. Several isotopomers of the complex were generated, and the IR spectra compared to results of density functional theory calculations. Other dimers between 1 and water were not found under these conditions. QM/MM calculations in simulated argon matrices reveal that an OH···p complex is unstable even at a time scale of picoseconds. This finding has implications on the related interaction between the tyrosyl radical and the water in biological systems.
The phenoxyl radical 1 was generated in high yields by flash vacuum pyrolysis of allyl phenyl ether 2 with subsequent trapping of the products in argon at 3 K. In water-doped argon matrices, an OH···O complex between 1 and water is formed that could be characterized by IR spectroscopy. Several isotopomers of the complex were generated, and the IR spectra compared to results of density functional theory calculations. Other dimers between 1 and water were not found under these conditions. QM/MM calculations in simulated argon matrices reveal that an OH···p complex is unstable even at a time scale of picoseconds. This finding has implications on the related interaction between the tyrosyl radical and the water in biological systems.