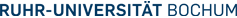Aminoacids and peptides
The conformation of peptides is governed by hydrogen bonds and other weak non-covalent bonds. To investigate the dynamics of conformational changes of peptides we have synthesized model peptides containing photolabile triggers such as disulfide bridges or azobenzene units. These systems were investigated by time resolved IR spectroscopy on a nanosecond scale in our laboratory and on the picosecond scale in cooperation with Prof. Hamm, University of Zürich.
The photochemical cleavage of the disulfide group triggers conformational changes which are observed by time resolved IR spectroscopy.
Solvation of Tetrapeptides
Solvent-Enhanced Conformational Flexibility of Cyclic Tetrapeptides
N. Berger, L. J. B. Wollny, P. Sokkar, S. Mittal, J. Mieres-Perez, R. Stoll, W. Sander. E. Sanchez-Garcia, ChemPhysChem 20 (2019), 1663.
Solvent and temperature can affect the structural properties of cyclic peptides by controlling their flexibility. Here, we investigate two cyclic peptides, featuring beta turns. Using temperature-dependent NMR and FT-IR, we observed a pronounced temperature effect on the conformation of the cyclic peptide D-1 in CHCl3 but a much smaller effect in CH3CN. Almost no effect was observed for its diastereomer L-1 within a similar temperature range and using the same solvents. With the aid of Replica Exchange Molecular Dynamics simulations and Quantum Mechanics/Molecular Mechanics calculations, we were able to explain this behavior based on the increased flexibility of D-1 CHCl3 in terms of intramolecular hydrogen bonding. The largest temperature dependence is observed for D-1 in CHCl3, while the temperature effect is less pronounced for L-1 in CHCl3 and for both peptides in CH3CN. This work provides new insights into the role of the environment and temperature on the conformations of cyclic peptides.
Read our paper in ChemPhysChem
Conformation and Dynamics of a Cyclic Disulfide-Bridged Peptide: Effects of Temperature and Solvent
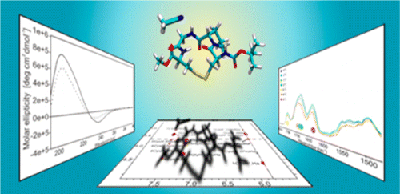
Conformation and Dynamics of a Cyclic Disulfide-Bridged Peptide: Effects of Temperature and Solvent
F. Li, K. Bravo-Rodriguez, C. Phillips, R. W. Seidel, F. Wieberneit, R. Stoll, N. L. Doltsinis, E. Sanchez-Garcia, W. Sander, J. Phys. Chem. B 117 (2013), 3560-3570.
The cyclic disulfide-bridged tetrapeptide cyclo(Boc-Cys-Pro-Gly-Cys-OMe) (1) was designed as a model for the study of solvent-driven conformational changes in peptides. The three-dimensional structure and dynamics of 1 were studied using a variety of experimental and computational techniques.
Read our publication in J. Phys. Chem. B