Carbocations and Carbenes
Fluorenyl Cation

The Fluorenyl Cation
P. Costa, I. Trosien, M. Fernandez-Oliva, E. Sanchez-Garcia, W. Sander, Angew. Chem. Int. Ed. 54 (2015), 2656-2660.
The fluorenyl cation is a textbook example for a 4p antiaromatic cation. However, contrasting results have been published on how the annelated benzene rings compensate the destabilizing effect of the 4p antiaromatic five-membered ring in its core. Whereas previous attempts to synthesize this cation in superacidic media resulted in undefined polymeric material only, we herein report that it can be generated and isolated in amorphous water ice at temperatures below 30 K by photolysis of diazofluorene. Under these conditions, the fluorenylidene is protonated by water to give the fluorenyl cation, which could be characterized spectroscopically. Its absorption in the visible-light range matches that previously obtained by ultrafast absorption spectroscopy, and furthermore, its IR spectrum could be recorded. The IR bands in amorphous ice very nicely match predictions from DFT and DFT/MM calculations, suggesting the absence of strong interactions between the cation and surrounding water molecules.
Read our publication in Angew. Chem. Int. Ed.
Phenyl Cation
Synthesis of the phenylcation via ionization of matrix-isolated phenyl radicals.
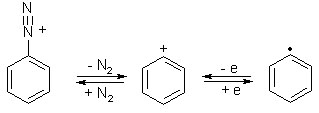
The phenylcation is mentioned in all textbooks of organic chemistry. However, only recently it could be isolated in condensed phase using the matrix isolation technique.