H2/O2 BIOFUEL CELLS
Redox enzymes may play a role as substitute for noble metal in devices for conversion of chemical fuel to electricity. The main issue is the stability of the biocatalysts. We have shown that the electron mediator can be designed to protect the catalyst from various degradation processes to achieve stability over weeks unter constant turn-over conditions for H2 oxidation in biofuel cell1.
We apply our expertise in chemistry and electrochemistry to develop novel strategies to protect sensitive catalysts from the harsh conditions encountered in fuel cells and other technological devices under operation2. To date only materials based on noble metals reached technological applications. In cooperation with W. Schuhmann, O. Rüdiger and W. Lubitz, we designed a redox hydrogel that serves as a protecting matrix for the catalyst3. The concept was successfully demonstrated with a hydrogenase as model for an efficient hydrogen oxidizing catalyst with extreme sensitivity. The hydrogel concept, reported in Nature Chemistry, opens the possibility to integrate a variety of other sensitive biological or artificial catalyst for which the intrinsic stability cannot be further increased. This is a major step toward a novel fuel cell redesign, which may reposition them at the forefront in the race toward global sustainable energy supply.
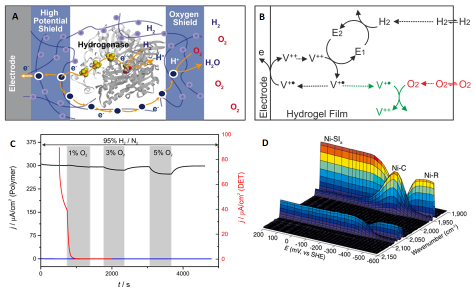
- Plumeré N., Rüdiger O., Alsheikh Oughli A., Williams R., Vivekananthan J., Pöller S., Schuhmann W., Lubitz W.
Nature Chemistry, 2014, 6, 822–827.
DOI: 10.1038/nchem.2022 - Alsheikh Oughli A., Conzuelo F., Winkler M., Happe T., Lubitz W., Schuhmann W., Rüdiger O., Plumeré N.
Angew. Chem. Int. Ed., 2015, 54 (42), 12329–12333.
DOI: 10.1002/anie.201502776 - Fourmond V., Stapf S., Li H., Buesen D., Birrell J., Olaf R.; Lubitz W., Schuhmann W., Plumeré N., Léger C.
J. Am. Chem. Soc., 2015, 137, 5494-5505.
DOI: 10.1021/jacs.5b01194

