Teilprojekt C: Prof. Dr. M. Havenith-Newen
Ruhr-Universität Bochum
High resolution IR-spectroscopy of molecular aggregates at ultra cold temperatures
The focus of our work lies on the investigation of molecular aggregates in suprafluid Helium nanodroplets with high resolution IR-spectroscopy. The unique infrastructure in Bochum, namely high resolution, highly sensitive, mass selective IR-depletion-spectrometer (resolution: 0.001 cm-1), provides a powerful tool for the research topics of the “Forscherguppe”. The already existing intensive cooperation in the “Forscherguppe” takes advantage of the combination of various spectroscopic and theoretical methods.
In the following we will focus on the two important aspects:
- Influence of D- and F- substitution on aggregation of pyridine complexes
The work of Boese et al. (subproject A) showed interesting results indicating changes in aggregation under H/D-substitution which will be investigated systematically by us with respect to the structure of pyridine dimers.
From previous microwave studies it is known that partially F-substitution at different ring positions specifically affects the geometry and charge distribution of pyridine [14]. The zero point vibrational energy is influenced by H/D-substitution. Both effects on the aggregation of the complexes will be investigated. As a starting point we will study the asymmetric C-H stretching mode of the monomer as well the dimer of pyridine at a region of 3200 cm-1. In addition, we will use high resolution, mass selective IR-spectroscopy techniques.
This project is directly connected with projects A and G. The intensive cooperation with the group of Dr. Merz guarantees a quick supply of partially D/F-substituted pyridine derivates for spectroscopy.
We will record IR-spectra of single aggregates in order to answer the following essential questions:
a) Which conformers are stable?
b) Which bands can be assigned to the dimer? Are they red- or blue shifted compared to the monomer?
c) Can we obtain rotational constants of the dimers by recording the rotational substructure of single vibrational bands?
d) How do the observed dimer structures agree with predicted structures observed in a matrix or crystal structure?
e) Which general rules can we deduce for the aggregation of cluster taking into account their charge distribution? What changes do we expect in case of isotopic substitution?
- Microsolvation of acids
Our first goal will be the further investigation of aggregation induced auto dissociation of HCl in water aggregates HCl-(H2O)n. Our most recent measurements show the complexity of that system. We would like to proceed with vibrational transitions in the H3O+ band for clusters with n=2, 3 or higher than 4.
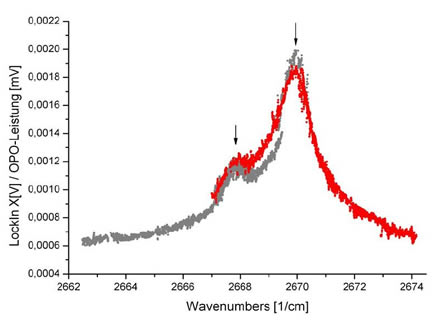
Spectrum of (HCl)n-(H2O)m-Clusters at low HCl pressures and medium water pressure. There are two lines at about 2270 and 2668 cm-1 (marked with an arrow). At these positions IR selective mass spectra were recorded.
We would like to investigate the dissociation mechanism of HCl-H2O aggregates in dependence of the pick-up sequence. Furthermore these investigations will be extended to microsolvation of amino acids like arginine or phenylalanine. Of special interest are investigations of the transition from neutral, canonical form to the zwitterionic structure. Our goal is to find out what the minimum number of water molecules is which is needed to form and stabilize the zwitterionic structure as recently discussed for the arginine monomer.
Helium nanodroplet spectroscopy is a perfect tool to study the aggregation of ultra cold monomers. This method allows the characterization of structures beyond the global minimum. Successive doping implies that each monomer is precooled to 0.37 K before aggregation. At large distances the monomers are prealigned due to dipole-dipole-orientation, preferentially in head-tail configuration. If this structure corresponds to a local minimum on the PES (potential energy surface), they might be trapped in this local minimum. In most cases the low temperature of 0.37 K prevents to surpass any barrier due to a lack of thermal activation energy. Similar effects can be observed in the matrix spectroscopy (subproject G, Sander), however in a matrix temperatures of up to 25-30 K will be reached (less in Ne matrices). Our long term goal is to understand the molecular processes which govern ultra cold chemistry.