Principles of electron microprobe analysis
During the measurements conducted in our lab, a sample is bombarded with a beam of electrons. The interaction of the electron beam with the sample material results in diverse effects, formation of secondary electrons, backscattered electron, X-rays and cathodoluminescence. These effects can be measured and used to make statements about the nature of the sample, e.g. chemical composition, structure, crystal orientation,...
In the following, the underlying physical processes and the analytical posibilities are briefly described.
Structure of an electron microprobe
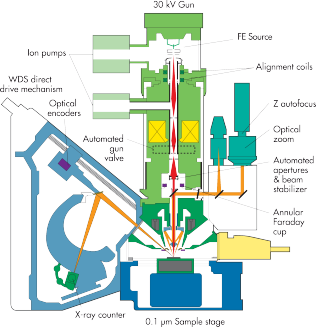
In the electron microprobe, an beam of electrons is generated in the electron gun. This is achieved with either a Schottky field emitter (SXFiveVE) or by heating a tungsten filament (SX50). An anode accelerates the electrons under vacuum towards the sample using high voltages (up to 30kV). A set of electron-optical lenses serves focusing the beam. The X-rays generated can be analysed using wavelength-dispersive spectrometers or an energy-dispersive detector. Further effects derived from interaction of the electron beam and the sample, such as formation of secondary and backscattered electrons as well as cathodoluminescence are used for imaging purposes. An additional optical unit allows for observing the sample in transmitted and reflected light. For navigation with micrometer precision, the sample is mounted on a motor-controlled stage.
Electron-specimen interaction
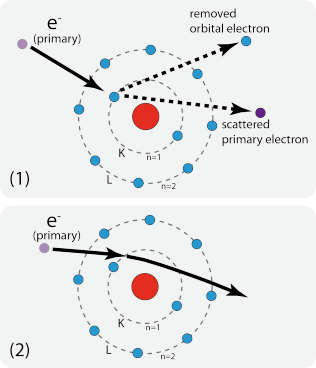
Under the influence of electron bombardment, two major interaction mechanisms with the sample are of importance. One type of inelastic interactions is removal of an orbital electron of the sample material by a primary electron. Subsequent filling of this "electron hole" with outer orbital electrons, leads to formation of X-rays with discrete wavelengths. As the electron configuration is characteristic for different elements, also the X-rays produced are 'characteristic X-rays'. A primary electron that does not collide with an orbital electron decelerates by the Coulombic field associated with the sample atoms. Result of this inelastic scattering process is a continuous X-ray spectrum (Bremsstrahlung). This radiation of continuous energy forms the background for the characteristic X-ray lines.
Further interaction processes are release of secondary electrons and of backscattered electrons as well as the occurrence of cathodoluminescence.
Alltogether, the effects of electron-specimen interaction allow for imaging and for qualitative and quantitative chemical analysis of the sample material.
- Sample preparation: Follow this link for details about sample preparation...
- Analysis types: Click here for details about different analysis types...