Glaucoma
Glaucoma is a disease that leads to a chronic loss of retinal ganglion cells and their axons, which is accompanied by damage to the optic disc and the visual field. Increased intraocular pressure is one of the main risk factors for glaucoma, but the exact pathogenesis is still unclear. Among others, mechanical processes, ischemic damage, excitotoxicity or immunological processes are discussed as disease triggers. Model systems are needed to reproduce and study this multifactorial disease.
EXPERIMENTAL AUTOIMMUNE GLAUKOMA MODEL
To investigate possible pressure-independent glaucoma mechanisms, our group works with a so-called experimental autoimmune glaucoma model (EAG). In this model, a loss of retinal ganglion cells is induced by immunization with ocular antigens (e.g. optic nerve homogenate, HSP27 or S100B).
The model was originally established and researched in rats. In order to expand the research possibilities, the model was transferred from the rat model animal to the mouse in cooperation with the Chair of Cell Morphology and Molecular Neurobiology (Ruhr University Bochum) (Reinehr et al. 2019).
In the EAG model, we have so far investigated various components of the immune response and subsequent cell death mechanisms. We have shown that immunization with ocular antigens leads to a loss of retinal ganglion cells and degeneration of the optic nerves (Laspas et al. 2011, Joachim et al. 2009,Grotegut et al. 2020,). Interestingly, immunization leads to a proliferation of glial cells in the retina and optic nerve even before retinal ganglion cells die (Casola et al. 2015, Noristani/Kuehn et al. 2016). In addition, autoantibody deposits have been found in the retina (Laspas et al. 2011), which activate the complement system, for example (Reinehr et al. 2016, 2018).
Based on glial and complement activation, various therapeutic approaches have also been developed in our working group in recent years.
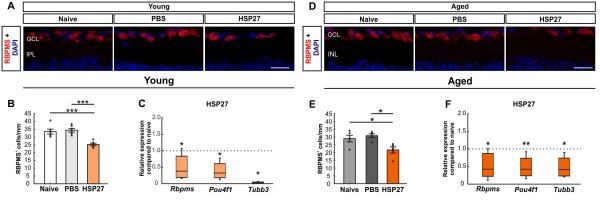
Figure 1: Effect of age in the EAG model. (A) RGCs in the retina of young mice were labeled with RBPMS (red), while DAPI (blue) was used to counterstain the cell nuclei. (B) RBPMS cell counting revealed a significant reduction in RGC number in the HSP27 group compared to the naive and PBS groups (both p<0.001). (C) A significant downregulation of Rbpms, Pou4f1 and Tubb3 mRNA expression was found in the HSP27 group compared to the naive and PBS mice (all p<0.050). (D) RGCs of aged mice were labeled with RBPMS (red), while DAPI (blue) was used to counterstain the cell nuclei. (E) The number of RGCs was significantly reduced in aged HSP27 mice compared to naive and PBS mice (both p<0.050). (F) HSP27 mice showed significant downregulation of Rbpms (p<0.050), Pou4f1 (p<0.010), and Tubb3 (p<0.050) compared to control retinas. Scale bar: 20 µm. Immunohistochemical values are mean±SEM and each symbol represents a single data point. RT-PCR values are median±quartiles±min/max. Dotted lines in C and F represent values of the respective control groups. *p<0.050, **p<0.010, ***p<0.001. Abbreviations: GCL=ganglion cell layer, IPL=inner plexiform layer, INL=inner granular layer, RGZ=retinal ganglion cells (Erb/Reinehr et al. 2023).
PRIMARY OPEN ANGLE GLAUCOMA (betaB1-CTGF)
The main risk factor for the development of glaucoma is increased intraocular pressure. The so-called βB1-CTGF high-pressure glaucoma model makes it possible to investigate this risk factor. The overexpression of CTGF (= Connective Tissue Growth Factor) leads to a stiffening of the trabecular meshwork and thus to an obstruction of aqueous humor outflow. One advantage of this model is that it increases intraocular pressure without external surgical manipulation.
Significant loss of optic nerve fibers was observed as early as 4 weeks of age (Junglas et al. 2012). In our working group, we were also able to show that after 15 weeks, the intraocular pressure of the animals is also increased and the number of ganglion cells is significantly reduced (Reinehr et al. 2019). This model therefore seems well suited to analyzing possible pressure-dependent mechanisms that also occur in human glaucoma.
COMBINATION MODEL OF EAG und βB1-CTGF MODEL
Since glaucoma is a multifactorial disease, the aim was to develop a model system that combines the risk factor of high pressure with the factor of the immunological component.
For this purpose, the βB1-CTGF model was combined with the EAG model by giving βB1-CTGF mice an additional intraperitoneal injection of bovine optic nerve homogenate (ONA). Six weeks after immunization, the mice of the combination model were found to have a significantly lower number of retinal ganglion cells and a more pronounced degeneration of the optic nerve (Reinehr et al. 2023, Kluge and Reinehr 2024). The combination model is therefore ideally suited to further deciphering the multifactorial pathogenesis of glaucoma.

