PI: Steen Rasmussen, P.-A. Monnard, SDU
At the FLinT Center at SDU, we are using our dual expertise (experimental in Interface Dynamics and theoretical in Numerical analysis and Simulations) to develop chemical supramolecular structures that can be manipulated in a chemical IT matrix.
Specifically we are using tensiometry, equilibrium dialysis and microscopy coupled with other quantitative methods to design and develop the necessary programmable and reconfigurable interfaces and compartments for chem-IT. We have been characterizing the association of (i) several modified DNA bioconjugates (in collaboration with the group of Andreas Herrmann) and (ii) photosensitizers centered around a Ruthenium complex (8-oxoganuanine-ruthenium trisbipyridine [= 8-oxoGRu] based on our collaborative work with LANL [1]) with interfaces such as phospholipid and fatty acid vesicles, as well as oil-in-water emulsions.
(i) Modified DNA bioconjugates with interfaces. All three interfaces have shown positive association with the modified DNA bioconjugates, with a clear quantitative advantage for those molecules that exhibit higher hydrophobicity relative to the hydrophilic DNA attached to them (Fig. 1A).
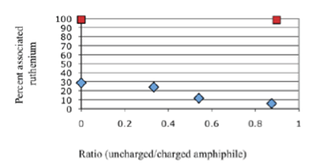
(ii) Modified photosensitizers. It was established that electrostatic interactions between the compartment surfaces and the positively charged 8-oxoGRu could permit its accumulation at the negatively charged surface of fatty acid vesicles, but 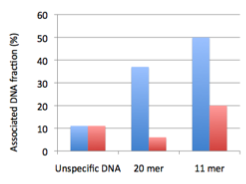 with low yields. In contrast, the addition of a hydrophobic chain on one of the ligand (bipyridine C-10 [= 8-oxoGRuC10:0]) was necessary to obtain a strong, long-lasting, almost quantitative interaction (Figure 1B) which was independent on the vesicle surface charge. A)
with low yields. In contrast, the addition of a hydrophobic chain on one of the ligand (bipyridine C-10 [= 8-oxoGRuC10:0]) was necessary to obtain a strong, long-lasting, almost quantitative interaction (Figure 1B) which was independent on the vesicle surface charge. A)
B)
Figure 1 Interactions of two systems with the interface of various amphiphile structures in water. A) Association of DNA bioconjugates with vesicles: (blue bars: POPC liposomes, red bars oleic acid vesicles) The unspecific double-stranded DNA was approximately 500 bp in length. The 20 mer was forming a hairpin with two hydrophobic chains (10 carbons in length) linked to two Uridine nucleobases located in the loop. The 11 mer was a shortened version of the 20 mer which could not form the double strand of the hairpin stem. B) Effect of the total surface charge on ruthenium complex association with fatty acid vesicles: (blue diamonds: 8-oxoGRu and in red squares 8-oxoGRuC10:0) The systems were tested for the interactions with decanoic acid/glycerol monodecanoate vesicles (10 carbon fatty acid). The total surface decreased as the fraction of non-ionic glycerol monodecanoate was increased.
The next issue was to determine whether the interactions with the various interface influence the catalytic activity of the systems (Figure 2).
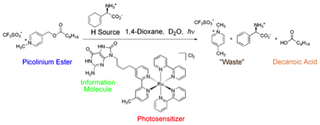
A)
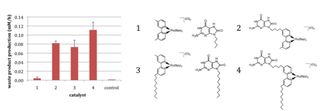
B)
Figure 2. Investigation of the ancillary photochemical reaction in vesicles. A) Reaction scheme for the photochemical production of amphiphile. The production of amphiphile (decanoic acid) by the photochemical cleavage of the picolylester precursor was detected. B) Initial rates of amphiphile production at the interface of vesicle Fatty acid vesicles were present in all four systems. In system 1 both information molecule and ruthenium metal were present separately as an aqueous species (aqueous intermolecular reaction), in 2 an aqueous complex composed of both an information and ruthenium part was used (aqueous intramolecular reaction), in 3 both information molecule and ruthenium metal were linked by an hydrophobic anchor to the container, but remained separated (linked intermolecular reaction) and in a hydrophobic information and ruthenium complex (linked intramolecular reaction).
Simultaneously, the photocleavage reaction was tested with emulsion compartments with systems 2 and 4 (Fig. 2). It was established that for each compartment types conditions could be found that supported the SDU photochemistry.
Thus, the alteration of the compartments required to fulfill the tasks proposed in ECCell project would not preclude our reactions. We are now designing a photocatalyst that incorporates DNA oligomers as electron relays, as well as the molecules needed to perform a nucleic acid replication based on the same photochemistry.
As a method of controlled supramolecular assembly, we are also developing at our SDU nano center, a new methodology of producing microfluidic devices rather quickly (Fig. 3). We will use such devices for the controlled assembly of liposomes, vesicles and emulsions containing the necessary modified DNA bioconjugates as discussed above.

Figure 3. Quick design to microdevice system. A) The microfluidic architecture is designed on a computer and printed in large A0 format. B) The design is photographed. C) The negative is used to make the microscale template using photoresist. D) The microdevices are made using the templates and standard PDMS technology.
For the simulation and theory contributions of SDU see here.
[1] M. S. DeClue, P.-A. Monnard, J. Bailey, S.E. Maurer, G.E. Colin, H. Ziock, J. W. Boncella, S. and Rasmussen Nucleobase Mediated, Photocatalytic Vesicle Formation from an Ester Precursor Molecule. J. Am. Chem. Soc., 131, 931-933, 2009.
