ZNA-Preparation
Recent subjects of investigation tended to the development of an isoelectric replication system. The system would consist of DNA and positively charged hybrid molecules as precursor molecules in a replication step. For the replication circle, the separation of the ingredients is to be achieved by migration in an electric field.
Preexperiments on the mobility of oligonucleotide-oligospermine conjugates (Zip Nucleic Acid; ZNA)[1] in an electric field under pH-variation were planned. The electrophoretic mobility of ZNA and ZNA-DNA double-strands and the pH-dependency was to be investigated.
In our group we focused on the preparation, purification and characterisation of fluorescence-labeled or unmodified ZNA and its corresponding counter-strand.
For the preparation of ZNA the fully protected spermine-phosphoramidite 1 was synthesized, according to literature. [1]
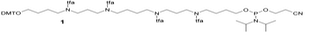
Figure 1: Structure of the DMT spermine phosphoramidite [1]
In solid-phase oligonucleotide synthesis the spermine 1 can be introduced at the 3´-end, the 5´-end or within the oligonucleotide sequence.
The synthesized oligonucleotide sequence of the ZNA was a 10mer sequence, derived from literature for utilization of the melting properties presented therein. [1] We introduced four spermine units at the 5´-end to compensate or over-compensate the negative charges of the phosphate-backbone at a suitable pH-value (Figure 2).

Figure 2: synthesized ZNA sequences and counterstrand.
For the hybridisation and electrophoreses experiments in microfluidics we additionally synthesized ZNA carrying fluorescent dyes at the 3´- or 5´-end. All syntheses were purified by RP-HPLC and characterized by MALDI-TOF.
Thiol-oligonucleotide ligation
To utilize the templated ligation of 3´- and 5´-thiol-oligonucleotides is one of our mayor efforts in the project. A FRET-quencher system for online measurements was designed and reaction kinetics for non-complementary, complementary and self-complementary sequences were examined for various sequences, concentrations, pH-values and temperatures. We found the templated reaction to be a fast and effective method for DNA ligation with little background reactions at micromolar concentrations.[2]
DNA additives
In replication experiments with complementary or self-complementrary sequences we observed strong background reactions due to precursor complexes. To reduce or overcome the side reactions caused by precursor complexes we synthesized a number of oligonucleotide sequences as additives to suppress side reactions.
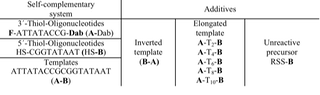
Table 1: Hybridisation additives for the suppression of an non-templated ligation.
The intention was to avoid a special proximity of the reactive sites by a favoured hybridisation of the precursor molecules to the additives. The strategies for the additives included the synthesis of an inverted template sequence, template sequences with a gap and also an unreactive disulfide precursor sequence as a dummy (Table 1).
The first experiments revealed only a small effect on the ligation reaction for high additive concentrations in case of the inverted sequence and for the T-elongated sequences. Concerning the size of the gap, no significant influence on the ligation was detected so far. Experiments with a closed gap by an inserted Apoly counter-strand and with an unreactive precursor are still in progress.
Single point substitution experiment
The templated ligation and its selectivity towards slight template modifications were to be demonstrated. Therefore we synthesized a template with a G vs T substitution.
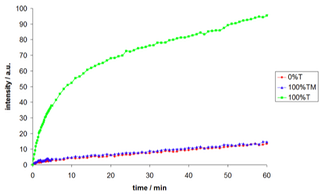
Figure 2: Influence of template on the ligation; reaction conditions: 0.5µM conc.;pH 7, 0.1M NaCl, 30°C.
T: TACTTCAAGCGGTATAAT; TM: TACTTCAAGCTGTATAAT.
The ligation experiments indicated no significant effect of the modified template on the ligation. A mutation close to the ligation site seems to have a big effect on the complex formation under the chosen conditions. Due to the high reaction rates and the sensitive FRET-detection method the single point mutation analysis is a promising application for the system. Nevertheless, further experiments with various modified sequences have to be performed.
Alternative ligation site
The disulfide ligation motif is based on a 5´-dC and a 3´-dG oxygen substitution by thiol. In a model the disulfide bridge appeared to be a structurally suitable substitution of the phosphordiester bond. Structural variations of the binding side are expected to have positive and negative consequences for the ligation caused by sterical hindrance or changed distances in the complex. In this context we changed the ligation site from the described system to a
5´¬dC¬-dxG-3´ system with identical sequences. We here expected changed properties for the reaction caused by bond length or product-template complex stability. Surprisingly, first experiments with a 3´-activated system indicated no relevant influence on the templated ligation. For a detailed comparison of both ligation sites further examinations under various conditions are planned.
[1] R. Noir, M. Kotera, B. Pons, J. Remy, J.P. Behr, J. AM. CHEM. SOC. 2008, 130, 13500–13505.
[2] V. Patzke, PhD thesis, Ruhr-Universität Bochum, 2005 http://www-brs.ub.ruhr uni bochum.de/netahtml/HSS/Diss/PatzkeVolker/Inhaltsverzeichnis.pdf.
