Fluorescence
Fluorescence is a sensitive and versatile spectroscopic property that can be used for the studies of biomolecules. Experimentally it can be used by either exploiting the intrinsic fluorescence of proteins that arises from the side chains of certain amino acids, such as tryptophan, or by introducing small fluorescence labels at defined positions within the protein. In recent years the green fluorescent protein (GFP) has been used to visualize other proteins within the living cell and even in the whole organism. We use those options to study the interaction of proteins - with respect to thermodynamics (affinity and specifity) and kinetics (association and dissociation rates). For this purpose we apply a variety of different methods, including conventional fluorescence spectroscopy, stopped flow and temperature jump relaxation. Of particular importance for our studies is the Förster resonance energy transfer (FRET). It enables us to study the intramolecular folding, or the binding of two biomolecules and gives valuable information on kinetics and distances.
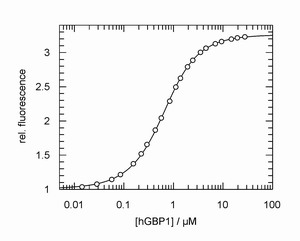
Left: Typical example of a fluorescence titration experiment to determine the affinity of the GTP-binding protein hGBP1 and a nucleotide. From the experiment the equilibrium constant can be obtained with high accuracy.
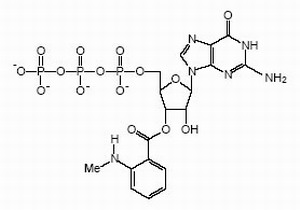
Right: Structural formula of GTP with a fluorescent "mant"-group that we use frequently in our studies.
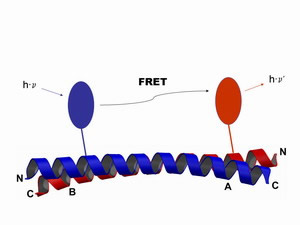
Schematical illustration of the Förster resonance energy transfer. Two different fluorophores are attached to a specific position within the same protein, or at two different proteins respectively. The excitation of the donor (blue) leads to the emission of a photon from the acceptor (red) in a strict distance dependent manner. From a FRET experiment we can obtain informations on the interaction equilibria, as well as on dynamic processes and structural changes.
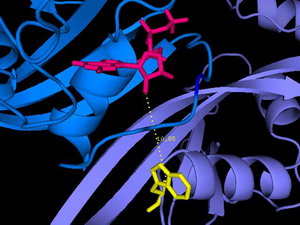
In diesem Ausschnitt aus der Röntgenstruktur des Ras/Nore Proteinkomplexes (entscheidend an der Regulation des programmierten Zelltods beteiligt) ist die räumliche Anordnung der Fluorophore (Nukleotid oben, Tryptophan unten) zu sehen, deren FRET die Interaktion von Ras und Nore wie auch den Abstand im Komplex quantitativ analysieren lässt.

