Catalytic addition reactions
Ru-catalyzed addition of amides to alkynes
The enamide moiety is an important substructure often encountered in biologically active compounds and synthetic drugs. Furthermore, enamides and their derivatives are versatile synthetic intermediates for the preparation of chiral amines, amino acids and various heterocyclic compounds. Traditional syntheses of this important substrate class involve rather harsh reaction conditions such as high temperatures and/or the use of strong acids and bases.
We have now developed a broadly applicable protocol for the synthesis of enamides, N-alkenyl carbamates, N-alkenyl ureas, and N-alkenyl lactames via a novel, waste-free, catalytic addition of N-nucleophiles to terminal alkynes.
The choice of ligands and additives determines the regiochemical outcome so that with two complementary catalyst systems, both the (E)-anti-Markovnikov products and the (Z)-anti-Markovnikov products can be synthesized highly regio- and stereoselectively.
Key references:
| 1. | |
| 2. | |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. | |
| 8. |
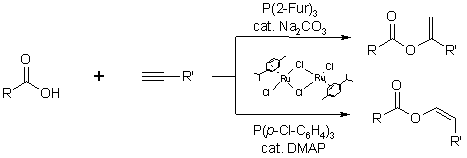
Ru-catalyzed addition of carboxylic acids to alkynes
We also developed new ruthenium catalysts for the addition of carboxylic acids to alkynes. These allow a preparatively simple,
highly regioselective access to either the alk-1-en-2-yl esters or the (Z)-alk-1-en-1-yl esters.
Key references:
| 1. |
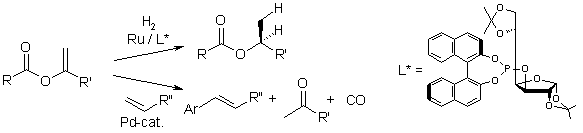
Asymmetric reduction of enol esters
In a cooperation with Prof. Reetz, we investigated the synthetic utility of alk-1-en-2-ylesters. In this context, we found that they can be reduced in a highly enantioselective fashion in the presence of a ruthenium catalyst bearing novel sugar-based chiral phosphite ligands.
In reductions of the type shown, e.e. values of more than 95 % were achieved, a new record for this substrate class. Thus, a synthetic sequence consisting of the addition of a carboxylic acid to an alkyne, followed by an asymmetric hydrogenation of the enol ester, appears to be a valuable alternative to the asymmetric reduction of dialkyl ketones (i.e. methyl ethyl ketone) followed by esterification
Key references:
| 1. |