Mechanistic investigations
In parallel to our experimental work, we also investigate the mechanism of palladium-catalyzed coupling reactions in general, and of our new reactions in particular, by means of in situ spectroscopy and DFT calculations. This work is performed in close cooperation with Prof. W. Thiel.
Mechanism of Cu/Pd-catalyzed decarboxylative cross-couplings
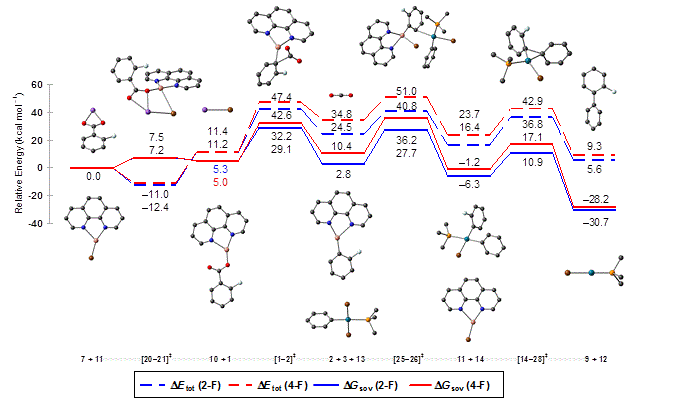
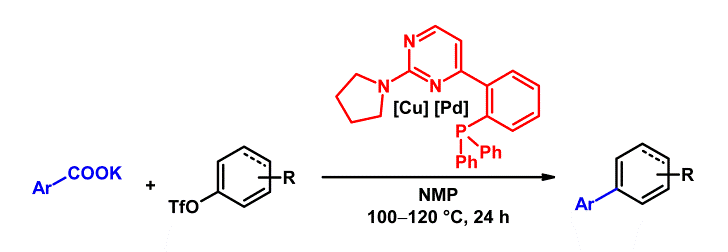
The reaction mechanism of the decarboxylative cross-couplings of benzoates with aryl halides to give biaryls, which is cooperatively catalyzed by palladium/copper systems, was investigated with DFT methods. The geometries and energies of all starting materials, products, intermediates and transition states of the catalytic cycle were calculated for the two model reactions of potassium 2- and 4‑fluorobenzoate with bromobenzene in the presence of a catalyst system consisting of copper(I)/1,10‑phenanthroline and the anionic monophosphine palladium complex [Pd(PMe3)Br]–. Several neutral and anionic pathways were compared, and a reasonable catalytic cycle was identified.
The key finding is that the transmetalation has a comparably high barrier as the decarboxylation, which was previously believed to be solely rate-determining. The electronic activation energy of the transmetalation is rather reasonable, but the free energy loss in the initial Cu/Pd adduct formation is high. These results suggested that research aimed at further improving the catalyst should target potentially bridging bidentate ligands likely to assist in the formation of bimetallic intermediates. Experimental studies confirm this somewhat counterintuitive prediction. With a bidentate, potentially bridging ligand, designed to support the formation of bimetallic adducts, the reaction temperature for decarboxylative couplings was reduced by 70 °C to only 100 °C. Furthermore, the presence of bimetallic Cu-Pd complexes was confirmed by ESI-MS investigations, suggesting that their formation is no longer rate-determining.
Key References
- A. Fromm, C. van Wüllen, D. Hackenberger, L. J. Gooßen, J. Am. Chem. Soc. 2014, 136, 10007-10023: Mechanism of Cu/Pd-Catalyzed Decarboxylative Cross-Couplings: A DFT Investigation. >>DOI
- D. Hackenberger, B. Song, M. F. Grünberg, S. Farsadpour, F. Menges, H. Kelm, C. Groß, T. Wolff, G. Niedner-Schatteburg, W. R. Thiel, L. J. Gooßen, ChemCatChem 2015, 7, 3579-3588: Bimetallic Cu/Pd Catalysts with Bridging Aminopyrimidinyl Phosphines for Decarboxylative Cross-Couplings at Moderate Temperatures. >>DOI
Oxidative addition of aryl halides to palladium catalysts
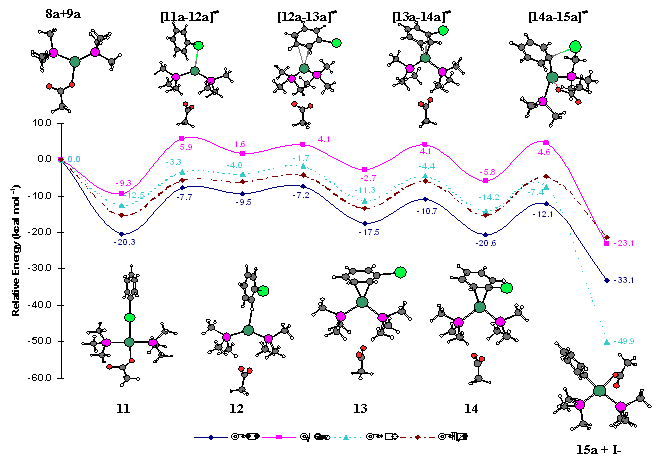
The oxidative addition of aryl halides to palladium(0) complexes under formation of aryl-palladium(II) species is the initiating step in numerous widely applied catalytic cross-coupling reactions, e.g. Suzuki reactions, Heck olefinations and Stille couplings. Unfortunately, the “textbook mechanism” provides no explanation for the pronounced influence that counter-ions of the Pd(II) pre-catalysts and added metal salts have on catalytic activities.
Based on DFT calculations, we propose a different mechanism for the oxidative addition of aryl halides to Pd-catalysts. Key intermediate is an anionic Pd-species in which the aryl halide coordinates to the palladium via the halide atom. This simple mechanism offers an alternative explanation for the complex experimental observations in cross-coupling reactions, without involving five-coordinate Pd-intermediates.
Key References
- L. J. Gooßen, D. Koley, H. Hermann, W. Thiel, Organometallics 2006, 25, 54-67: Palladium Monophosphine Intermediates in Catalytic Cross-Coupling Reactions: A DFT Study. >>DOI
- L. J. Gooßen, D. Koley, H. Hermann, W. Thiel, J. Am. Chem. Soc. 2005, 127, 11102-11114: The Palladium-Catalyzed Cross-Coupling Reaction of Carboxylic Anhydrides with Arylboronic Acids: A DFT Study. >>DOI
The Palladium-Catalyzed Cross-Coupling Reaction of Carboxylic Anhydrides with Arylboronic Acids
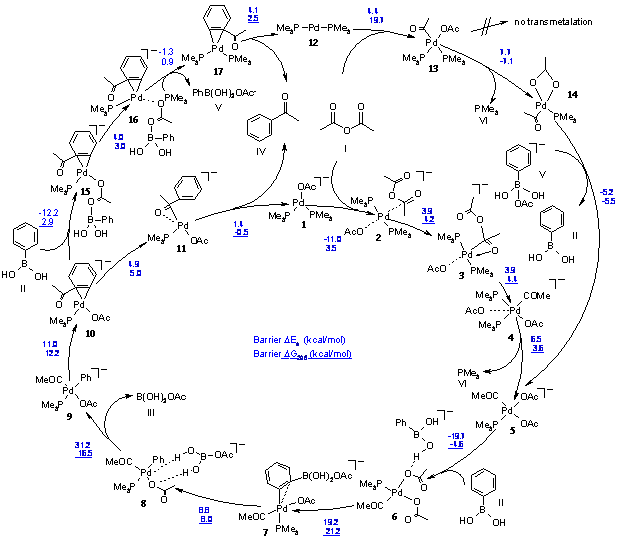
The mechanism of the cross-coupling of phenylboronic acid with acetic anhydride, a viable model of the widely used Suzuki reaction, has been studied by DFT calculations at the BP86/6-31G* level of theory. Two alternative catalytic cycles have been investigated, one starting from a neutral Pd(0)L2 complex, the other from an anionic “Jutand-type” [Pd(0)L2X]– species. The reaction profiles are in good agreement with the experimental findings, as both pathways require only moderate activation energies. Both pathways are dominated by cis-configured square planar palladium(II)diphosphine intermediates.
While the “textbook mechanism” mainly proceeds via trans-configured palladium(II)diphosphine complexes, cis-configured intermediates dominate in the calculated catalytic cycles. Moreover, according to the proposition of Amatore and Jutand, the anionic pathway involves five-coordinate species, whereas we find only four-coordinate intermediates, in qualitative agreement with related calculations on the oxidative addition of aryl halides to anionic palladium(0) complexes.
Key References
- L. J. Gooßen, D. Koley, H. Hermann, W. Thiel, Organometallics 2006, 25, 54-67: Palladium Monophosphine Intermediates in Catalytic Cross-Coupling Reactions: A DFT Study. >>DOI
- L. J. Gooßen, D. Koley, H. Hermann, W. Thiel, J. Am. Chem. Soc. 2005, 127, 11102-11114: The Palladium-Catalyzed Cross-Coupling Reaction of Carboxylic Anhydrides with Arylboronic Acids: A DFT Study. >>DOI