Diffusion of water on insulating surfaces
Understanding the basic mechanisms of surface processes is of technological importance. Among others, the solvation properties of water – the most commonly used solvent – is of high interest. The surface processes on insulators show relevance in catalysis, electronic switches or nano technology.
This project is a GIF-funded collaboration of the group of Gil Alexandrowicz (Technion, Haifa) and the chair of Karina Morgenstern (PC I, RUB).
In this project, the dynamic properties of water on insulator surfaces are investigated by two complementary methods, namely Helium spin-echo spectroscopy (HSE) and low-temperature scanning tunneling microscopy (LT-STM). The time-scale of both techniques differs by around 12 order of magnitude, allowing to measure the dynamics of water in a large temperature-range.
The insulators used in this project are NaCl and a metal oxide. First STM results of water on NaCl show that diffusion of water molecules can be detected at 45 K and can be quantified by the Arrhenius’ law.
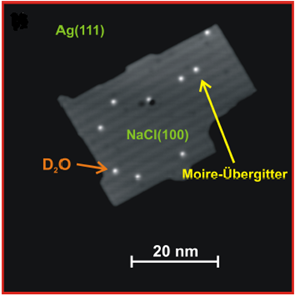
Fig. 1: STM image of D2O/NaCl(100)/Ag(111). On top of the NaCl island, single water molecules are visible. A Moiré lattice arises from the superposition of the NaCl lattice and the surface lattice (U = 502 mV, I = 30 pA, T = 5 K) [1].
[1] C. Bertram, Kinetik und Clusterbildung von Wasser auf Metallen und einem Isolator, Masterarbeit Leibniz Universität Hannover (2012)
Got interested?

