Inelastic electron tunneling spectroscopy at solvent related structures
Solvation is a main mechanism for chemical processes, as it determines synthesis and catalytic reactions and is a cutting edge in modern physical chemistry.
To understand solvation on a single molecules basis we use a bottom up approach. In this approach we add single water molecules to adsorbed species on a surface. As a model for solvation we study single molecules on surfaces surrounded by water molecules. We have shown before that the specific adsorption sites of these first water molecules at the solvated species can be studied by scanning tunneling microscopy (STM) [1]. In this study we propose to study the strength of the binding by inelastic electron tunneling spectroscopy (IETS). Both methods give information about the formation of solvation shells.
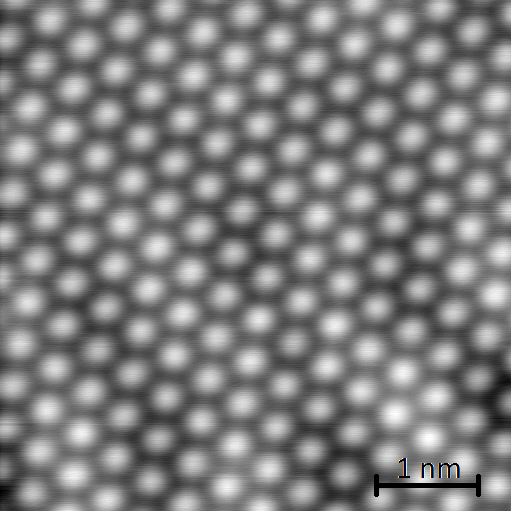
However, molecules adsorbed on surfaces change their orbital structure because of binding to the surface. For a better comparability to liquid phase solvation we plan to reduce this interaction by building up a Xenon spacer layer between bare surface and molecules. Molecules are expected to interact with this layer only by van-der-Waals forces. The orbital change will thus be minimized. We use STM and IETS to explore the influence of a Xenon layer between substrate and molecules on solvation, and compare these results with theoretical models.
Got interested? Want to know more about it? Contact via email:
martin.meyeraufderheide@rub.de
[1] Henzl J., Boom K., Morgenstern K. , J. Am. Chem. Soc., 2014, 136 (38), 13341–13347
Using the first steps of hydration for the determination of molecular conformation of a single molecule.
Fig.1: STM image of Xenon on Cu(111):