Looking deep into water
General Overview:
The low-temperature STM/AFM is a combination of an Atomic Force Microscope and a Scanning Tunneling Microscope. The combination of these two powerful scanning probe techniques with subatomic resolution allows us to observe the electronic and the atomic structure of the surface at cryogenic temperatures and manipulate single molecules and atoms. To make this possible the microscope is intensively protected against any kind of noise and cooled down to the temperature of liquid Helium.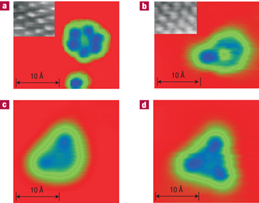
Colored STM images of an ice hexamer (a), heptamer (b), octamer (c), and nonamer (d) on Cu(111) and Ag(111) [Michaelides et al., Nat. Mat. 6 (2007) 597].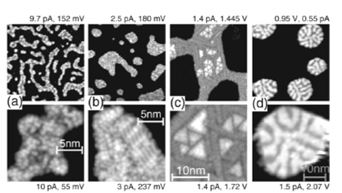
STM images of four different ice structures on Cu(111) formed by annealing to 118 K (a), 130 K (b), 140 K (c), and 149 K (d) [Mehlhorn et al., PRL 99 (2007) 246101]
Forever, water has played the central role for humankind in the world. In the modern world, water sustained its interest because of its very special characteristics and specifications. For example water is an important solvant in modern sciences. 60 years ago, ice revealed another secret: The solvation of electrons, whose mechanism is sketched in figure 3[I. Taub et al., Discuss. Faraday Soc. 36 (1963) 206; figure from U. Bowensiepen, Prog. Surf. Sci. 78 (2005) 87].
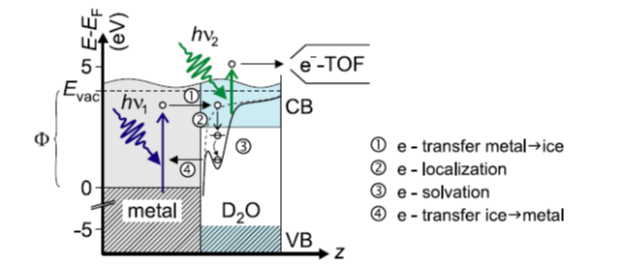
Schematics about generation and measurement/proof of solvated electrons within bulk D2O ice on a metal substrate. The first incoming laser photon excites an electron into the conduction band of the bulk ice (e-transfer metal -> ice). After several relaxation steps the electron relaxes into a trap within the bulk (e-localization). There it can solvate to the ground state of the ice trap (e-solvation). Before the electron relaxes to the metal again (e-transfer ice->metal), it can be excited with a second laser photon to get measured by a time of flight spectrometer [U. Bovensiepen, Prog. Surf. Sci. 78 (2005) 87].
Within a powerful project-team we investigate the usage of the electron solvation together with researchers of the Universities of Duisburg/Essen and Erlangen. We aim at the controlled use of these energetic electrons to aid reactions on the ice surface using a dissociation of halogenated benzene as the easiest test. To achieve this far-reaching goal we study also the rich structure variety of ice grown at low temperatures on different surfaces as the first step in this brand-new project within the RESOLV initiative of our beautiful university.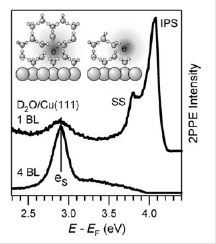
Further reading:
http://news.rub.de/wissenschaft/2017-01-20-chemie-wie-entstehen-filigrane-eiskristalle
Got interested? Want to know more about it? Contact cord.bertram@rub.de

