A 19F MAS and Electron Microprobe Study on structural incorporation and partitioning of Fluorine in Layer Silicates and Aluminosilicate Melts
In general fluorine presents a minority component in granitic rocks. By means of fractional processes it can be enriched in silicate melts as moderate incompatible element. Even small amounts of fluorine affect the chemical and physical properties of the melts as well as the phase relationships of partially molten granites. Besides the melt layer silicates like biotite, phlogopite and muscovite are the favourite phases, which intercalate fluorine on differentiation of magmas.
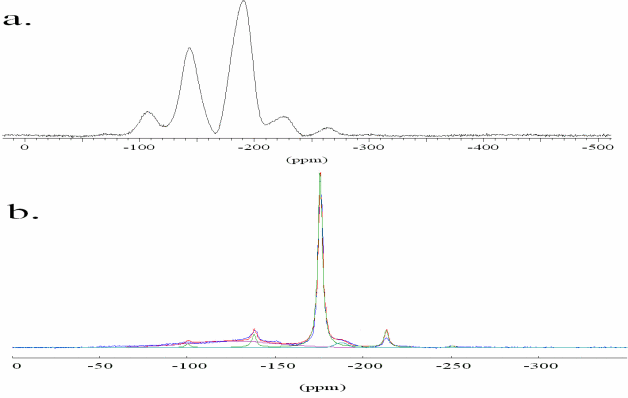
The partitioning behaviour is related to the stability of F species in the present phases. The structural incorporation of F in silica-rich aluminosilicate glasses and phlogopite is studied by MAS NMR using the spin one-half nucleus 19F which covers a wide area of chemical shift. In accordance with previous studies two structural species are identified in the F-containing glasses (Fig 10a). The signal at -193 ppm is assigned to AlF63- complexes, while the resonance at -143 ppm cannot be assigned ambiguously [22].
F-exchange experiments between hydrous alumino-silicate melts and phlogopite (Mg/(Mg+Fe) = 1) or biotite (Mg/(Mg+Fe) = 0.46) were performed at 1073 K and 1 kbar. Electron microprobe analyses show that F is preferentially incorporated in the layer silicate. Preliminary results indicate a higher distribution coefficient crystal/melt for phlogopite (16.5) than for biotite (2.5) at such conditions. The MAS NMR spectrum of the exchange experiment products display a sharp signal at -176 ppm due to Fluorine in MgF64- complexes in the phlogopite structure (Fig 10b) [23]. The two broad signals characterize the local structure of fluorine in the alumino-silicate melt as described above.
The relative amounts of fluorine in phlogopite and melt could be quantified after the experiments from the 19F MAS NMR spectra. Depending on the distinct starting mixtures of reaction components the resulting signal areas of the two components are different. Using aluminosilicate glasses containing 3.11 wt% F and pure OH-phlogopites in a mass ratio 3:1 70 free alumosilicate glasses and F-phlogopite in a mass ratio 3:1 19F MAS NMR indicates only 50% of the fluorine being incorporated in the phlogopite.


