Prof. Dr. Dr. h.c. Beate Brand-Saberi
Ruhr-Universität Bochum
Institut für Anatomie
Abteilung für Anatomie und Molekulare Embryologie
- Tel: +49 (0)234 32 27780
- Fax: +49 (0)234 32 14474

Forschung
Unsere Abteilung ist ein internationales Team aus Wissenschaftler*innen, Student*innen und Techniker*innen mit Interesse an Fragestellungen der Zell- und Entwicklungsbiologie und der Digitalisierung der anatomischen Lehre.
Instagram Feed
STEM CELL ENGINEERING
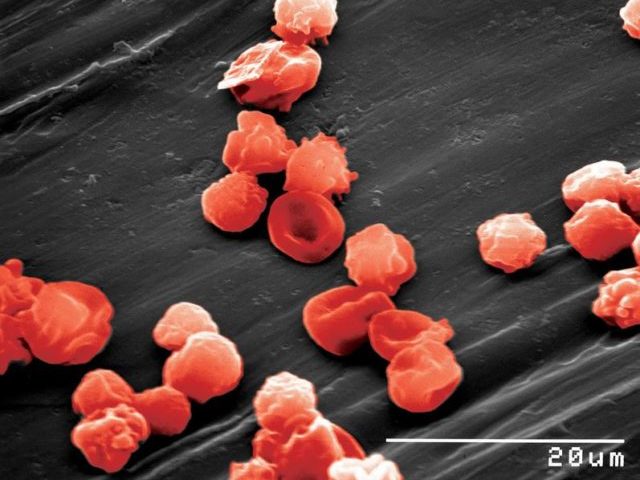
Somatic cells can be reprogrammed to a pluripotent embryonic stem cell-like state (induced pluripotent stem (iPS) cells) by combinations of the transcription factors OCT4, SOX2, NANOG, LIN28, KLF4 and cMYC (Takahashi et al., 2006, 2007; Yu et al., 2007; Park et al., 2007). The initial studies were underdone inducing pluripotency in mouse and human fibroblasts. We were able to demonstrate, that mouse and human neural stem cells which express SOX2, KLF4 and cMYC, can be reprogrammed to iPS cells by retroviral expression of OCT4 (Kim, Zaehres et al., 2008, 2009). Afterwards, we have comparatively evaluated the hematopoietic and erythroid as well as neural differentiation potential of human cord blood and neural stem cell derived iPS cells (Hargus et al., 2014, Dorn et al., 2015).
Patient-derived induced pluripotent stem cells and their differentiation derivatives hold great promise as novel in vitro model systems to study human pathogenesis. We are currently focusing to model neural (Frontotemporal Dementia) and skeletal muscle (Duchenne, LGMD) diseases with human iPS cells and CRISPR/Cas9 genome editing. Recently, we have developed a human skeletal muscle organoid differentiation system, which recapitulates myogenesis (Mavrommatis et al., 2020).
We are further developing our iSTEM (Molecular and Developmental Stem Cell Biology) master program since its inception in 2010 by incorporating state-of-the-art topics of stem cell research in the teaching modules.
NACHWUCHSARBEITSGRUPPE STRESS
Die Nachwuchsarbeitsgruppe Stress beschäftigt sich mit den Effekten der neuroendokrinen Stressreaktion und nähert sich den assoziierten Forschungsfragen sowohl auf empirischer als auch auf molekularbiologischer Weise. Hierzu nutzen wir physiologische Messungen, wie Herzratenvariabilität, Hormonbiologische- bzw. enzymatische Analyseverfahren und widmen uns der Fragebogenkonstruktion. Um pränatale Stressforschung zu modellieren, nutzen wir den Hühnerembryo unter Injektionen des Stresshormons Corticosteron und schließen dem histologische sowie molekularbiologische Methoden zur Analyse an.

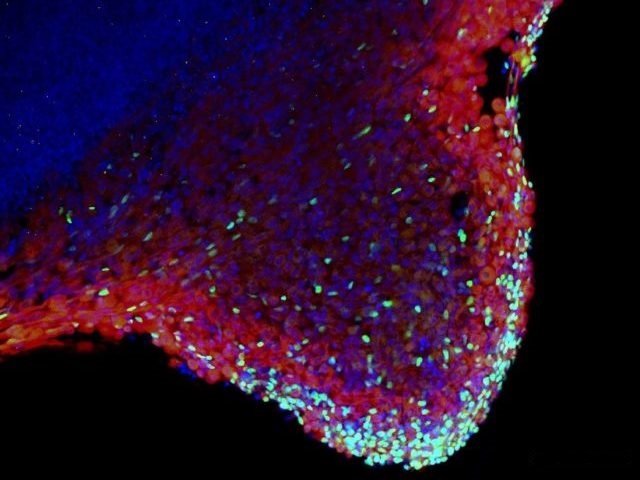
MYOGENESIS AND MYOPATHIES
Transcription factors are part of the machinery that decides which genes should be active and which silent. In this way, transcription factors have a strong impact on embryonic development. Skeletal muscle development is an excellent process to study the roles of transcription factors in vitro and in vivo. The development of skeletal muscle is a complex process which has been highly conserved in evolution. It involves cell specification, tissue rearrangement and differentiation (Buckingham 2001, 2006; Brand-Saberi 2005). These steps are all tightly controlled by a number of signaling molecules and transcription factors that have been put into an increasingly complex hierarchy during recent years. In this hierarchy, the paired box transcription factor Pax3 has been attributed a key role upstream of some of the important key factors of the bHLH family of myogenic regulatory factors (Tajbakhsh et al. 1997).
More recently, members of the sine oculis/Six gene family, Six1 and Six4 have been shown to have an important function in myogenesis upstream of Pax3 and the MRFs (Grifone et al. 2005). Six1 and Six4 encode homeoprotein transcription factors.
In the human, disturbances in skeletal muscle development and maintenance cause congenital myopathies that show a wide variety of clinical manifestations and progression of the symptoms. Quite often the aetiology of congenital myopathies remains obscure and the disorders are not yet curable by available therapies. The congenital myopathies are characterized by structural and histochemical anomalies such as muscle fibre disproportions or protein aggregations. The histological variety is in accordance with their clinical appearance, which ranges from mild to very severe myopathies with perinatal respiratory failure. Some known causes of myopathies include defects of membranous proteins, enzymes or structural proteins (Bornemann and Goebel, 2001). We have recently focused on the study of bHLH and other transcription factors which have been shown to play a decisive role in tissue specification during development.
ATOH8
ATOH8 in Zebrafish: „Small fry” with Great Perspectives
Small fry:
1. young children
2. young or small fishes
3. people/things regarded as unimportant
(collinsdictionary.com)
When it comes to zebrafish, there is no such thing as being “regarded as unimportant”. In the past decades, the popularity of the small fresh water fish as a model organism in medical and scientific research has risen enormously.
… got curious? Find out more about research opportunities with zebrafish
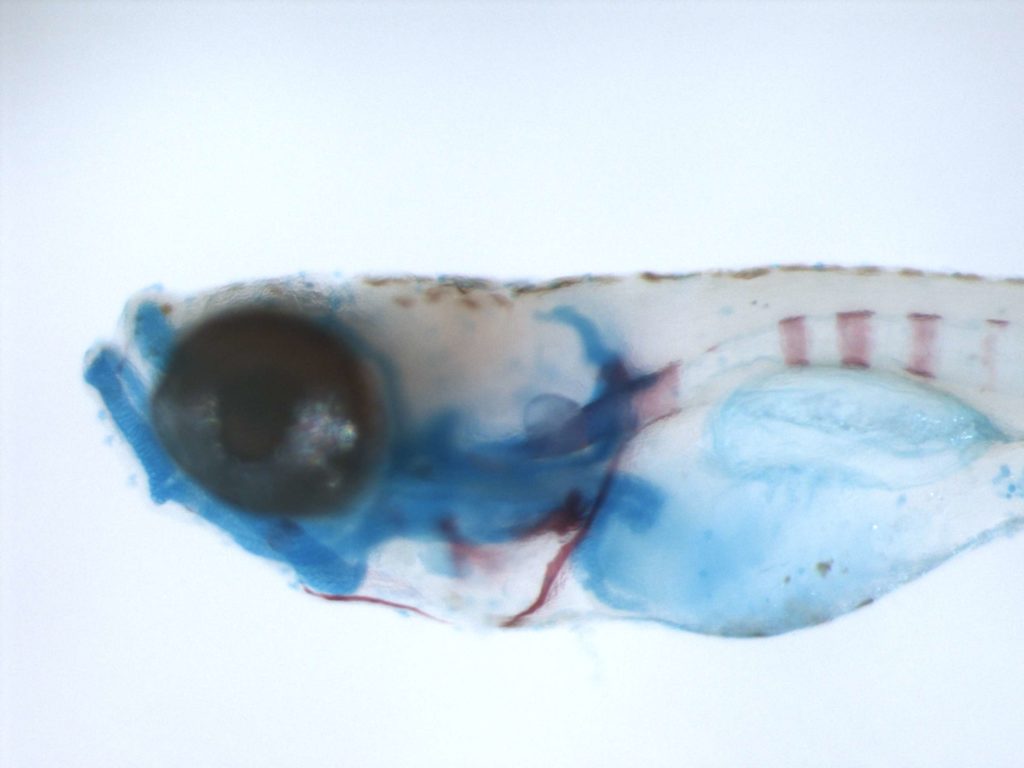
DEOXYRIBONUCLEASES
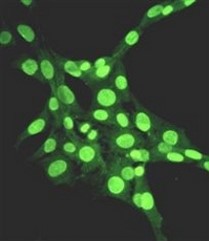
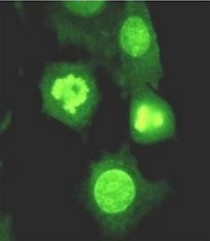
Extracellular nucleases, which occur in most body fluids of vertebrates, predominantly belong to a family of Ca2+ and Mg2+ (Mn2+)-dependent endonucleases, which was named by its first identified member, Deoxyribonuclease I (DNase I / DNASE1). So far three further members beside DNASE1 were found: DNASE1-like 1-3 (DNASE1L1-3).
Our group characterizes the physiological functions of these nucleases by employing gene-knockout mouse models. Especially the functions of DNASE1, which is highly expressed by glands lining the gastro- and urogenital tract and DNASE1L3 (DNase γ), which is expressed by macrophages and activated B-cells, are of our major interest. Both nucleases occur in serum of mammals and our experiments hint to their contribution in the suppression of anti-nuclear autoimmunity, which is characteristic for a human disease called Systemic Lupus Erythematosus (SLE). Both enzymes display a complementary substrate specificity – DNASE1 efficiently degrades protein-free DNA whereas DNASE1L3 preferentially hydrolyzes protein-complexed DNA (chromatin) – and appear to cooperate in the destruction of exogenous (microorganisms) and endogenous (primary or secondary necrotic cells) DNA. Impairment of these processes might lead to a prolonged and chronic exposure of the immune system to DNA-antigens leading to an increased probability for the breakdown of self-tolerance towards nuclear antigens. Indeed, SLE is regarded as a multigenetic disease caused by defects which impair the removal of dying cells and cell debris in the context of defects leading to a deregulated and hypersensitive immune system.
In summary, we intend to elucidate the role of extracellular nucleases in the removal of nuclear cell debris released under physiological and pathological conditions (necrosis) and the consequences resulting from an impairment of these processes.
Recent Publications
- Brendel M, Scharf M, Kindler U, Divvela SSK, Brand-Saberi B. Biology (Basel). 2023 Sep 19;12(9):1252. doi: 10.3390/biology12091252. PMID: 37759651 Detection of Math6-Expressing Cell Types in Murine Placenta.
- Gellisch M, Schäfer T, Yahya I, Joswig M, Cheng X, Morosan-Puopolo G, Brand-Saberi B. Eur J Investig Health Psychol Educ. 2023 Aug 12;13(8):1491-1504. doi: 10.3390/ejihpe13080109. PMID: 37623306 Rethinking Learning Experience: How Generally Perceived Life Stress Influences Students’ Course Perceptions in Different Learning Environments.
- Gellisch M, Bablok M, Morosan-Puopolo G, Schäfer T, Brand-Saberi B. Healthcare (Basel). 2023 May 26;11(11):1558. doi: 10.3390/healthcare11111558. PMID: 37297698 Dynamically Changing Mental Stress Parameters of First-Year Medical Students over the Three-Year Course of the COVID-19 Pandemic: A Repeated Cross-Sectional Study.
- Bablok M, Gellisch M, Scharf M, Brand-Saberi B, Morosan-Puopolo G. Ann Anat. 2023 Apr;247:152056. doi: 10.1016/j.aanat.2023.152056. Epub 2023 Jan 22. PMID: 36696929 Spatiotemporal expression pattern of the chicken glucocorticoid receptor during early embryonic development.
- Gellisch M, Bablok M, Divvela SSK, Morosan-Puopolo G, Brand-Saberi B. Biology. 2023; 12(5):656. https://doi.org/10.3390/biology12050656 Systemic Prenatal Stress Exposure through Corticosterone Application Adversely Affects Avian Embryonic Skin Development.
- Yahya I, Brand-Saberi B, Morosan-Puopolo G. Heliyon. 2023 Mar 3;9(3):e14230. doi: 10.1016/j.heliyon.2023.e14230. PMID: 36923876; PMCID: PMC10009738. Chicken embryo as a model in second heart field development.
- Bablok M, Gellisch M, Scharf M, Brand-Saberi B, Morosan-Puopolo G. Ann Anat. 2023 Jan 22;247:152056. doi: 10.1016/j.aanat.2023.152056. Online ahead of print. PMID: 36696929 Spatiotemporal expression pattern of the chicken glucocorticoid receptor during early embryonic development.
- Gellisch, M., Wolf, O. T., Minkley, N., Kirchner, W. H., Brüne, M., & Brand-Saberi, B. (2022). Decreased sympathetic cardiovascular influences and hormone-physiological changes in response to Covid-19-related adaptations under different learning environments. Anatomical sciences education, 15(5), 811–826. https://doi.org/10.1002/ase.2213
- Gellisch, M., Morosan-Puopolo, G., Wolf, O. T., Moser, D. A., Zaehres, H., & Brand-Saberi, B. (2023). Interactive teaching enhances students’ physiological arousal during online learning. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft, 247, 152050. Advance online publication. https://doi.org/10.1016/j.aanat.2023.152050
- Satya Srirama Karthik Divvela, Patrick Nell, Markus Napirei, Holm Zaehres, Jiayu Chen, Wanda Maria Gerding, Huu Phuc Nguyen, Shaorong Gao and Beate Brand-Saberi (2019). bHLH Transcription Factor Math6 Antagonizes TGF- β Signalling in Reprogramming, Pluripotency and Early Cell Fate Decisions. Cells 8, 529.
- Böing M, Brand-Saberi B, Napirei M. (2018). Murine transcription factor Math6 is a regulator of placenta development. SCIENTIFIC REPORTS 8(1), 14997.
- Chen A, Li J, Song L, Ji C, Böing M, Chen J, Brand-Saberi B (2017). GGNBP2 is necessary for testis morphology and sperm development. Scientific Reports 7, Article Number: 2998.
- Brand-Saberi B, Zaehres H (2016). The development of anatomy: from macroscopic body dissections to stem cell-derived organoids. Histochem Cell Biol. 146 (6), 647-650.
- Wang B, Balakrishnan-Renuka A, Napirei M, Theiss C, Brand-Saberi B (2015). Spatiotemporal expression of Math6 during mouse embryonic development. Histochem Cell Biol. 143 (6), 575-582.
- Mazur AJ, Morosan-Puopolo G, Makowiecka A, Malicka-Błaszkiewicz M, Nowak D, Brand-Saberi B (2014). Analysis of gelsolin expression pattern in developing chicken embryo reveals high GSN expression level in tissues of neural crest origin. Brain Struct Funct. Doi: 10.1007/s00429-014-0923-5 [Epub ahead of print]
- Lever M, Brand-Saberi B, Theiss C. (2014). Neurogenesis, gliogenesis and the developing chicken optic tectum: an immunohistochemical and ultrastructural analysis. Brain Structure and Function 219 (3), 1009-1024.
- Morosan-Puopolo G, Balakrishnan-Renuka A, Yusuf F, Chen J, Dai F, Zoidl G, Lüdtke THW, Kispert A, Theiss C, Abdelsabour-Khalaf M, Brand-Saberi B. (2014). Wnt11 Is Required for Oriented Migration of Dermogenic Progenitor Cells from the Dorsomedial Lip of the Avian Dermomyotome. PLOS ONE 9(3): e92679
- Pu Q, Abduelmula A, Masyuk M, Theiss C, Schwandulla D, Hans M, Patel K, Brand-Saberi B, Huang R. (2013). The dermomyotome ventrolateral lip is essential for the hypaxial myotome formation. BMC Developmental Biology, 13:37.
- Chen J, Dai F, Balakrishnan-Renuka A, Leese F, Schempp W, Schaller F, Hoffmann MM, Morosan-Puopolo G, Yusuf F, Bisschoff IJ, Chankiewitz V, Xue J, Chen J, Ying K, Brand-Saberi, B. (2011) Diversification and molecular evolution of ATOH8, a gene encoding a bHLH transcription factor. PLoS One 6(8):e23005
- Rehimi, R., Khalida, N., Yusuf, F., Morosan-Puopolo, G., Brand-Saberi, B. (2010) A novel role of CXCR4 and SDF-1 during migration of cloacal muscle precursors. Dev. Dyn. 239(6):1622-31
- Chen,J., Morosan-Puopolo, G., Dai, F. Wang, J., Brand-Saberi, B. (2010) Molecular cloning of chicken Cecr2 and its expression during chicken embryo development. Int.J. Dev Biol. 54(5):925-9
- Bonnet A, Dai FP, Brand-Saberi B, Duprez D (2009) Vestigial-like 2 acts downstream of MyoD activation and is associated with skeletal muscle differentiation in chick myogenesis. Mech. Dev. 127(1-2):120-36
- Hausott B; Vallant N, Yang L, Dai FP, Brand-Saberi B, Klimaschewski L (2009) Sprouty2 down-regulation promotes peripheral axon regeneration by adult sensory neurons in vitro. MCN 42(4):328-40
- Zhao W., Dai F., Bonafede A., Schäfer S., Jung M., Gamel A.J.,Yusuf F., Wang J., Brand-Saberi B. (2009) Histone deacetylase inhibitor, Trichostatin A, affects gene expression patterns during morphogenesis of chick limb buds in vivo. Cells Tissues Organs 190:121-134
- Vasyutina, E., Stebler, J., Brand-Saberi, B., Schulz, S., Raz, E., Birchmeier C. (2005) CXCR4 and Gab1 co-operate to control the development of migrating muscle progenitor cells. Genes and Development 19: 2187-2198.
- Dai, F.P., Yusuf, F., Farjah, G.H., Brand-Saberi, B. (2005) pEGFP-vector based RNA interference for in vivo gene functional analysis in chicken embryos. Dev. Biol. 285: 80-90
- Yusuf, F., Rehimi, R., Dai, F.P., Brand-Saberi, B. (2005) The expression pattern of the chemokine receptor CXCR4 in chick embryo development. Anat. Embryol. 210:35-41
- Hornik, C., Krishan, K., Yusuf, F., Scaal, M., Brand-Saberi, B. (2005) cDermo-1 misexpression induces dense dermis, feathers and scales. Dev. Biol. 277:42-50


